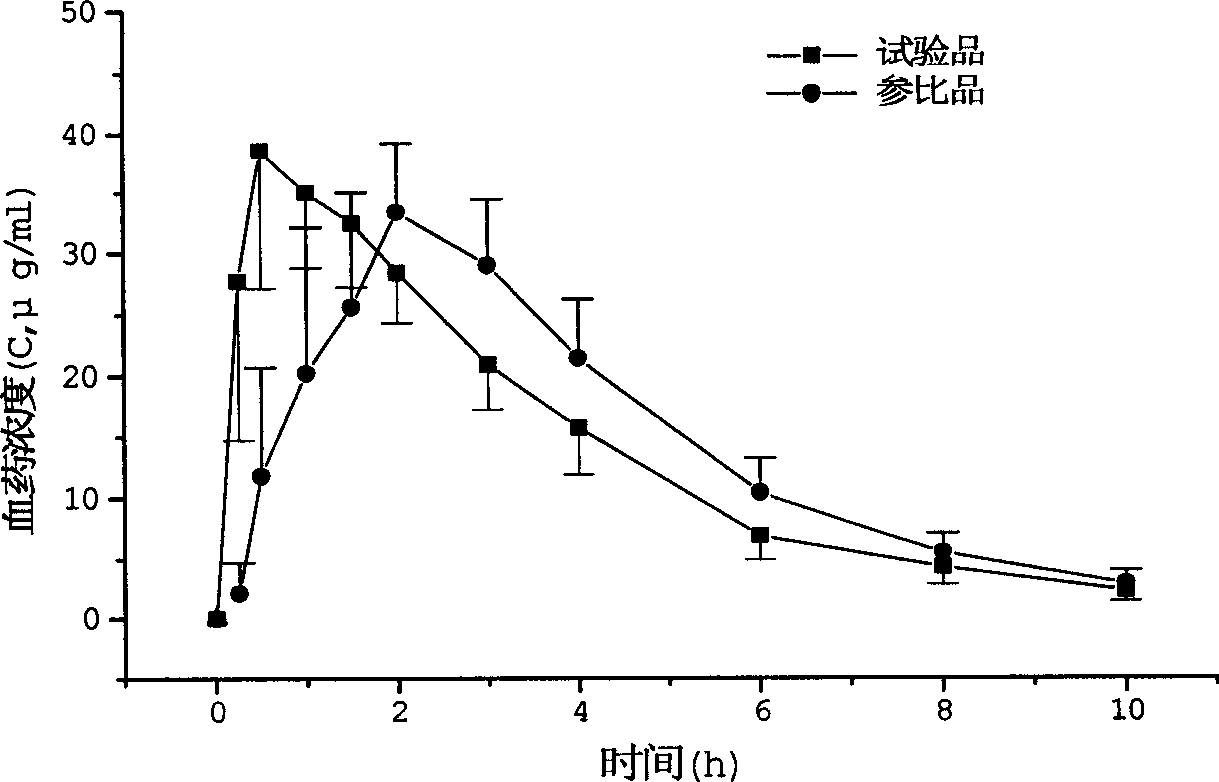
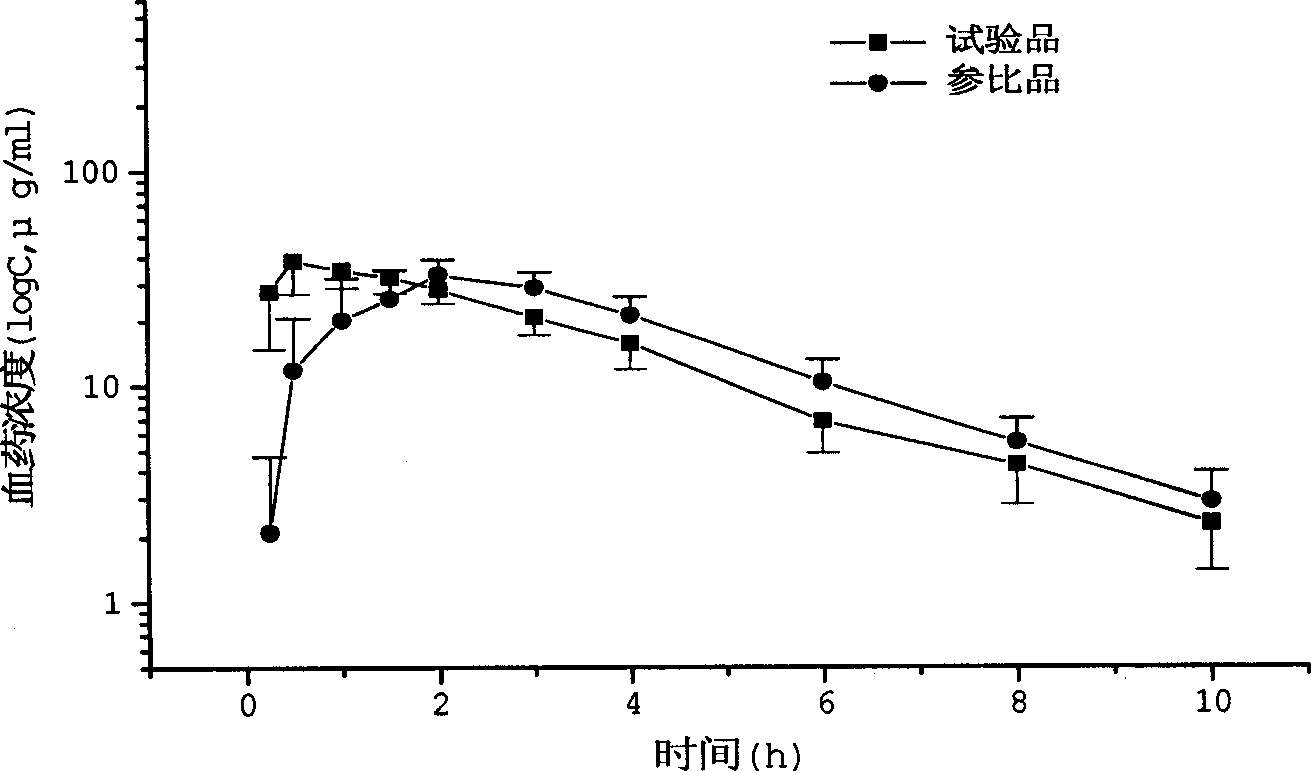
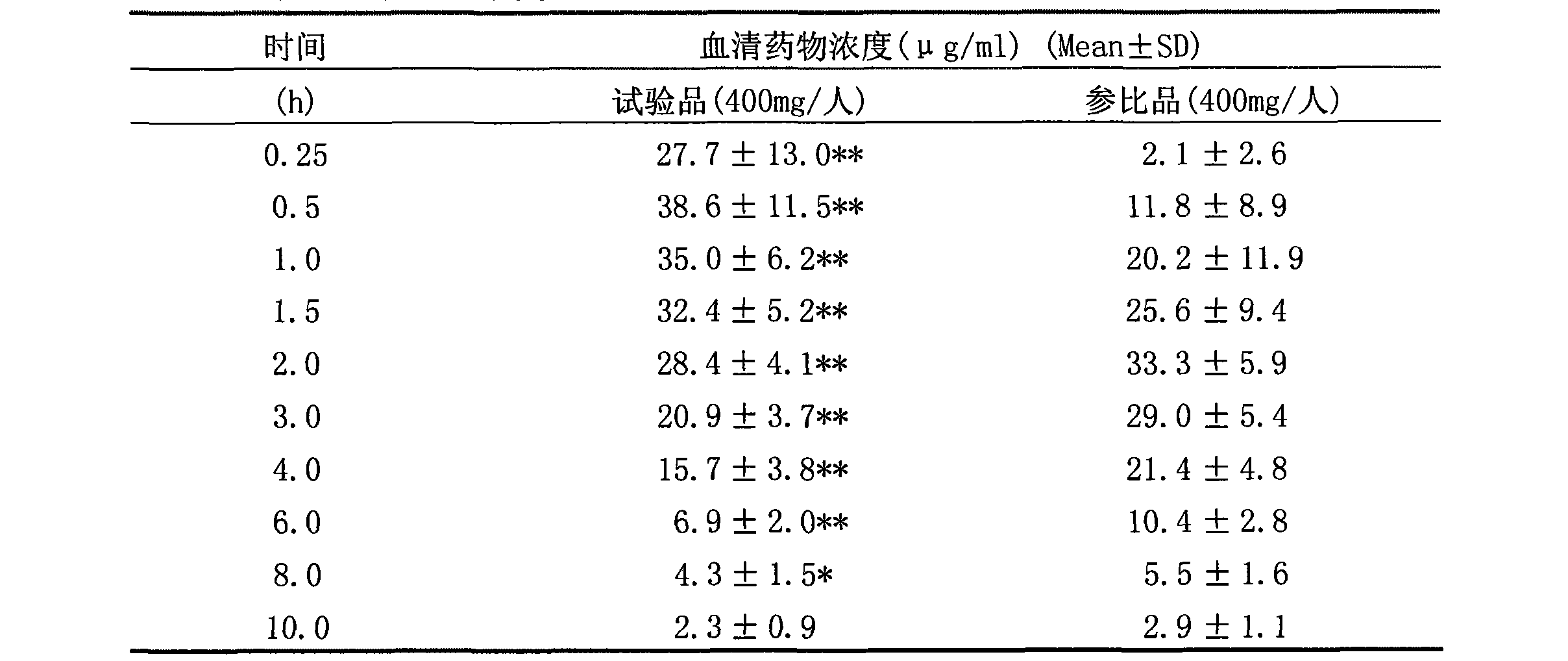
Arginine ibuprofen tablet and preparation method thereof
An arginine ibuprofen and tablet technology, which is used in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of large production and storage space, large proportion of excipients, poor patient tolerance, etc. problem, to achieve the effect of low cost, fast onset and easy portability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Every 1000 arginine ibuprofen tablet preparations, weigh materials according to the following formula:
[0037] Arginine Ibuprofen 370g
[0038] Starch 55.5g
[0039] 8% starch slurry appropriate amount
[0040] Magnesium Stearate 1.5g
[0041] The arginine ibuprofen tablet of the present invention is prepared by the raw and auxiliary materials taken according to the following steps:
[0042] 1. Sieve ibuprofen arginine and starch through a 100-mesh sieve and mix well;
[0043] 2. Take 8% starch slurry, add it to the mixture in step 1, and stir while adding to make a soft material;
[0044] 3. Pass the soft material through a 20-mesh sieve to make wet granules;
[0045] 4. Dry the wet granules at 50-60°C;
[0046] 5. Use a 20-mesh sieve to sieve the dry granules in step 4, add magnesium stearate, mix well, and press into tablets.
Embodiment 2
[0048] Every 1000 arginine ibuprofen tablet preparations, weigh materials according to the following formula:
[0049] Arginine Ibuprofen 370g
[0050] Microcrystalline Cellulose 80g
[0051] 5% hypromellose aqueous solution 60ml
[0052] Magnesium stearate 1.8g
[0053] The arginine ibuprofen tablet of the present invention is prepared by the raw and auxiliary materials taken according to the following steps:
[0054]1. Sieve ibuprofen arginine and microcrystalline cellulose through a 100-mesh sieve and mix well;
[0055] 2. Take 5% hypromellose aqueous solution, add it to the mixture in step 1, stir while adding, and make a soft material;
[0056] 3. Pass the soft material through a 20-mesh sieve to make wet granules;
[0057] 4. Dry the wet granules at 50-60°C;
[0058] 5. Use a 20-mesh sieve to sieve the dry granules in step 4, add magnesium stearate, mix well, and press into tablets.
Embodiment 3
[0060] Every 1000 arginine ibuprofen tablet preparations, weigh materials according to the following formula:
[0061] Arginine Ibuprofen 185g
[0062] Microcrystalline Cellulose 100g
[0063] Lactose 125g
[0064] Magnesium stearate 4.1g
[0065] The arginine ibuprofen tablet of the present invention is prepared by the raw and auxiliary materials taken according to the following steps:
[0066] 1. Arginine ibuprofen, microcrystalline cellulose, and lactose were passed through a 60-mesh sieve, and mixed;
[0067] 2. Take magnesium stearate, add it to the mixture in step 1, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com