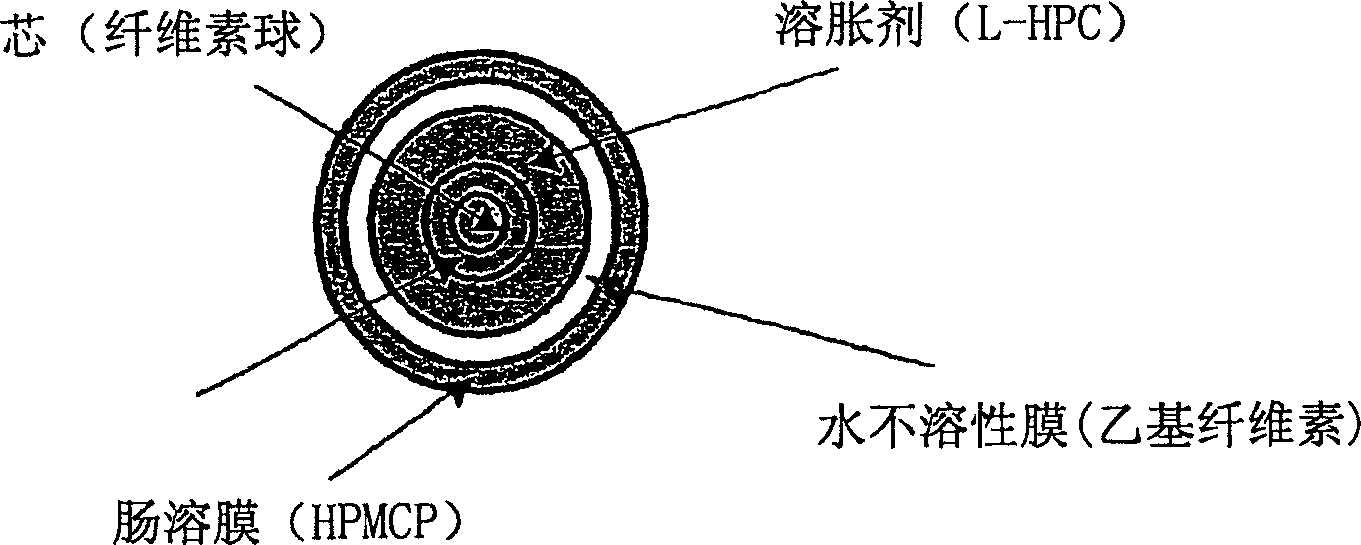
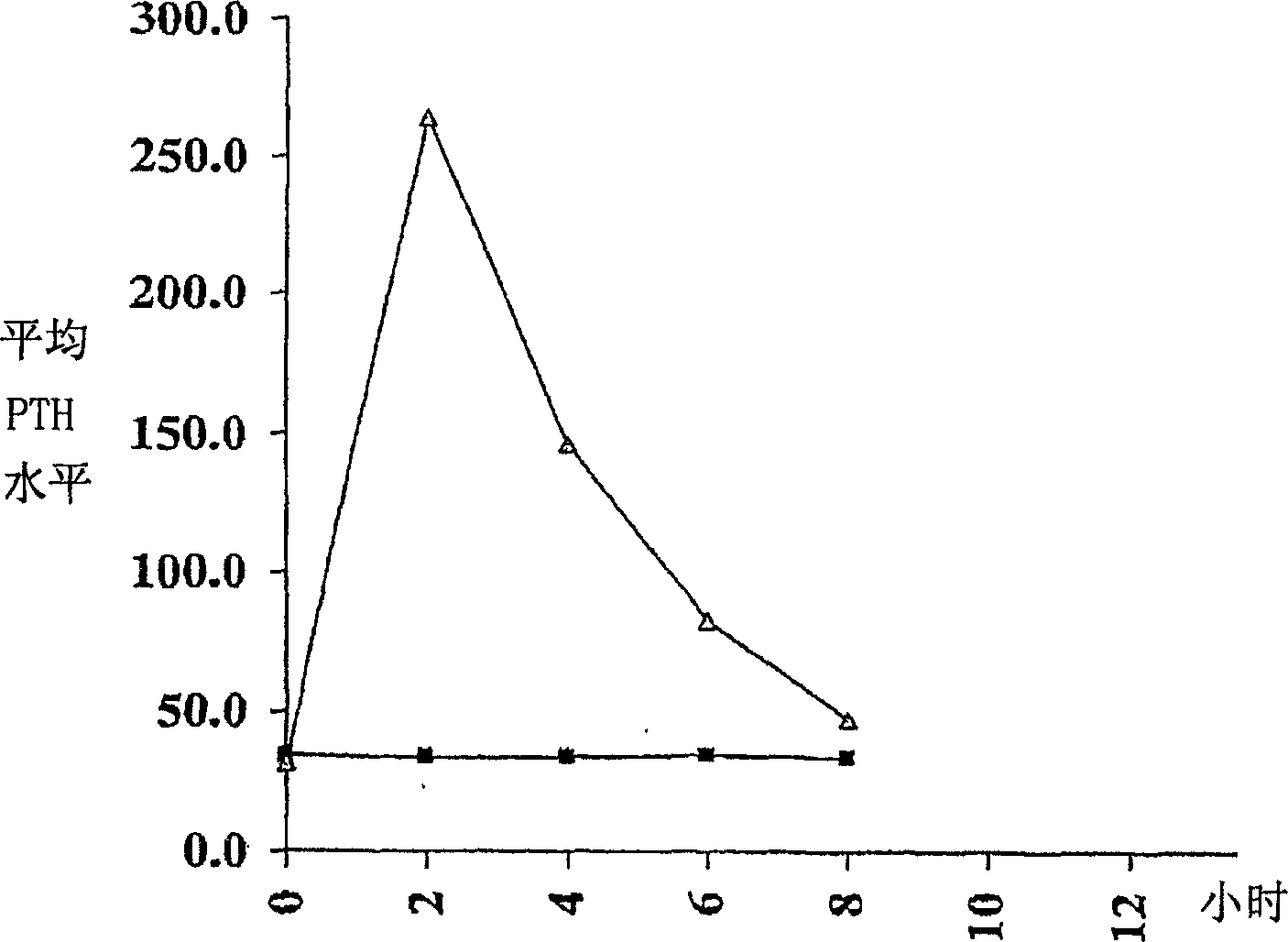
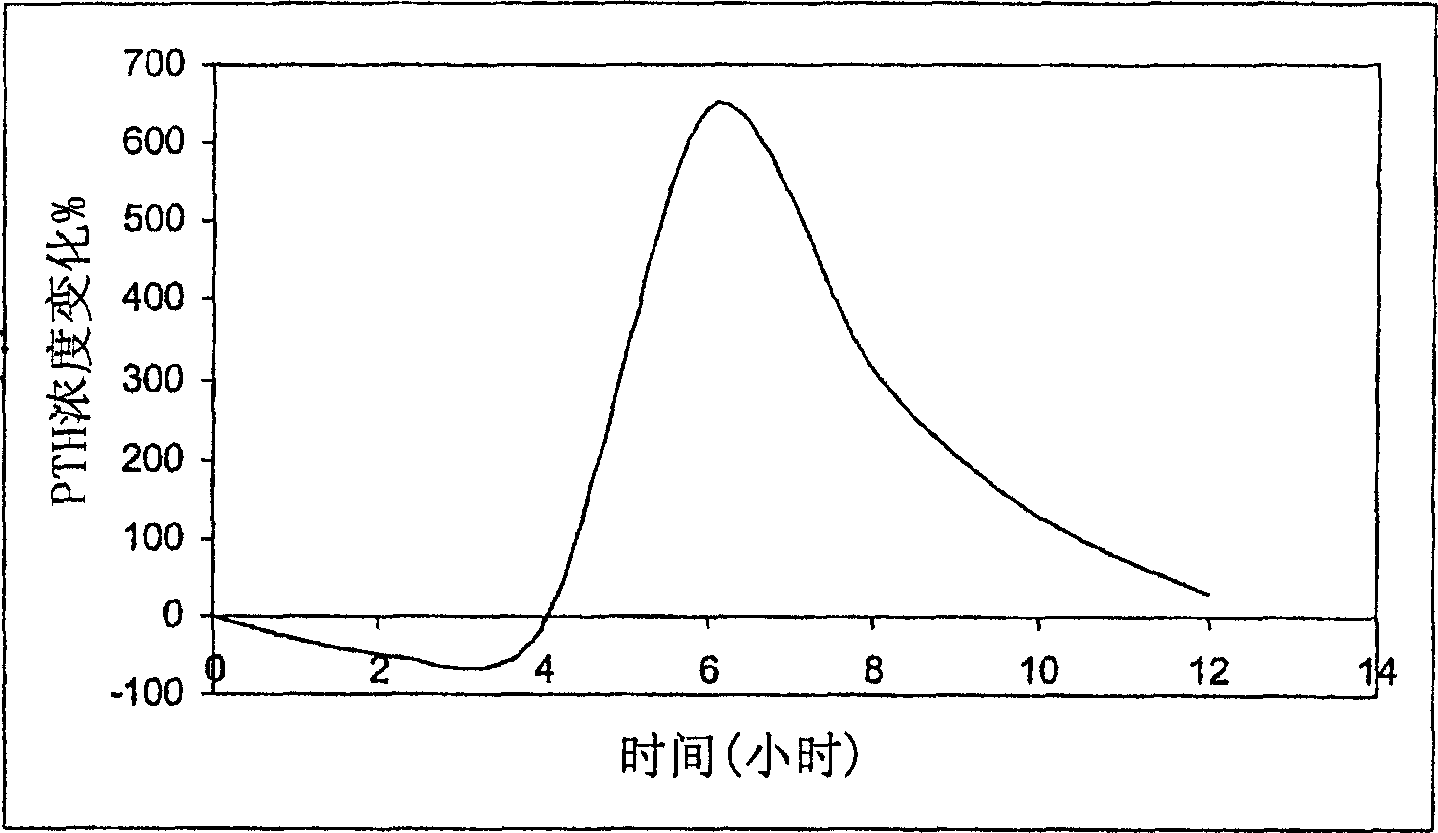
Parathyroid hormone (pth) containing pharmaceutical compositions for oral use
A composition and drug technology, applied in the direction of drug combination, sugar-coated pills, pharmaceutical formulations, etc., can solve the problem of slow release of active substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0414] Preparation of PTH-containing tablets for intestinal delivery (jejunum)
[0415] This example illustrates the preparation of tablets for intestinal delivery (jejunum). The composition of the tablets is shown in Table 1.
[0416] Table 1
[0417] Ingredient Quantity (g)
[0418] PTH (lyophilized PTH) 120.0
[0419] insulin inhibitor 1 600.0
[0420] Sodium Lauryl Sulfate 34
[0421] Microcrystalline cellulose 560.0
[0422] Sodium carboxymethyl cellulose 560.0
[0423] Polyvinylpyrrolidone 90 26
[0424] Magnesium Stearate 10.0
[0425] Talc 90.0
[0426] Total 2000.0
[0427] 1. Assuming an effective concentration of about 0.5 mg / ml, the maximum intestinal volume of a 100 cm intestine is about 500 ml. Burst release covers 20cm of intestine, that is, the required effective dose is 0.5mg / ml×500ml×0.2m=50mg / dose.
[0428] The components are mixed in a high shear mixer and the granules are wetted and dried in a fluidized bed to an absolute...
Embodiment 2
[0448] Preparation of PTH-containing tablets for intestinal delivery (ileum)
[0449] This example illustrates the preparation of tablets for intestinal delivery (ileum). The composition of the tablet is shown in Table 4.
[0450] Table 4
[0451] Ingredient Quantity (g)
[0452] PTH (lyophilized PTH) 120.0
[0453] Amatadine 2 31.8
[0454] sodium deoxycholate 3 720.0
[0455] Microcrystalline cellulose 500.1
[0456] Sodium carboxymethylcellulose 500.1
[0457] Polyvinylpyrrolidone 90 28
[0458] Magnesium Stearate 10.0
[0459] Talc 90.0
[0460] Total 2000.0
[0461] 2. Assuming an effective concentration of about 0.0265 mg / ml (50 μΜ), the maximum intestinal volume of a 100 cm intestine is about 500 ml. Burst release covers 20cm of intestine, that is, the required effective dose is 0.0053mg / ml×532ml×0.2m=0.56mg / dose.
[0462] 3. Calculated at 3% of the solid dosage form, not in the dissolved form.
[0463] Tablets were pre...
Embodiment 3
[0476] Preparation of PTH-containing cores for colonic delivery
[0477] This example illustrates the preparation of cores for colonic delivery. The composition of the core is shown in Table 6.
[0478] Cores were prepared using extrusion / spheronization techniques.
[0479] Table 6
[0480] Ingredient Quantity (g)
[0481] PTH (lyophilized PTH) 400.0
[0482] Aprotinin 4 250.0
[0483] EDTA 1000.0
[0484] Microcrystalline cellulose 337.5
[0485] Lactose monohydrate 462.5
[0486] Sodium carboxymethyl cellulose 50.0
[0487] Purified water 775g
[0488] 4. Assuming an effective concentration of about 0.25 mg / ml, the maximum intestinal volume for 100 cm of intestine is 500 ml. Burst release covers 20cm of intestine, that is, the required effective dose is 0.25mg / ml×500ml×0.2m=25mg / dose.
[0489] The components were mixed and wetted in a Fielder high shear mixer. The wetted mass was extruded in a Nica E 140 extruder with a 0.6 mm screen. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com