Cefuroxime axetil pharmaceutical composition and preparation method thereof
A technology of cefuroxime axetil and pharmaceutical preparations, which is applied in the field of medicine, can solve problems such as cefuroxime axetil not being tightly packaged, product taste decline, and taste decline, and achieve improved drug dissolution rate, good dissolution effect, and easy-to-obtain equipment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
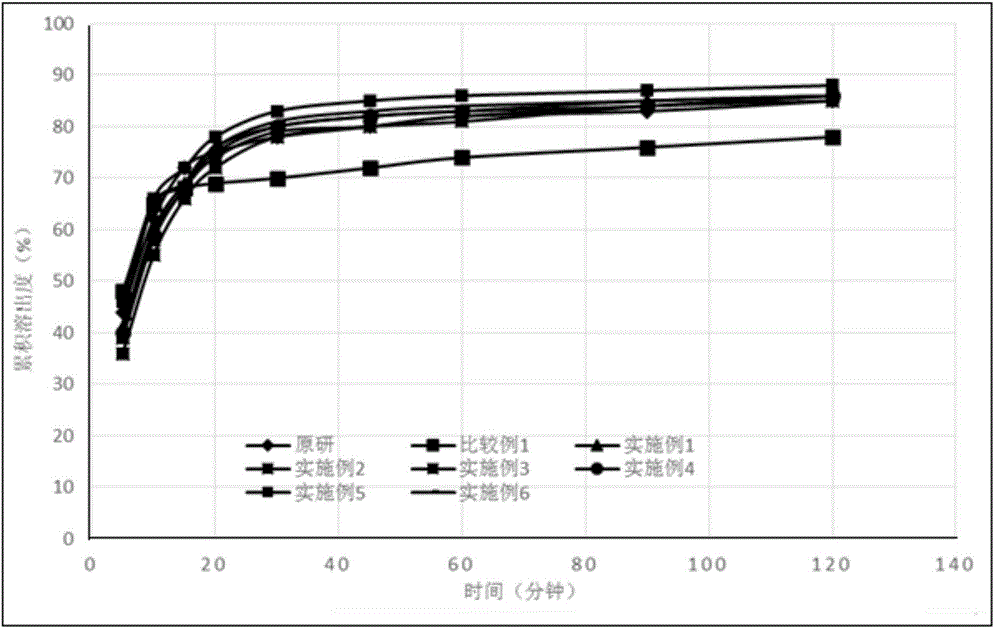
Image
Examples
Embodiment 1
[0040] Embodiment 1 (1000 bags of dry suspensions)
[0041]
[0042]
[0043] The preparation method is as follows:
[0044] (1) Glyceryl monostearate is heated and melted, and the melting temperature is 90°C.
[0045] (2) In the fluidized bed, adjust an appropriate air volume to fully fluidize the cefuroxime axetil raw material.
[0046] (3) In the fluidized bed, adjust the temperature of the material to 40°C to 50°C, and the atomization pressure to 0.1 to 0.3Mpa. Under the action of compressed air, the glyceryl monostearate is passed through the spray device to form atomized droplets, which are slowly sprayed on the surface of the fully fluidized cefuroxime axetil raw material, so that it is uniformly coated.
[0047] (4) Add sucrose, mannitol, low-substituted hydroxypropyl cellulose and hydroxypropyl cellulose to the above powder, granulate, add the remaining excipients after drying, mix and pack separately, and prepare a dry suspension.
Embodiment 2
[0048] Embodiment 2 (1000 bags of dry suspensions)
[0049]
[0050] The preparation method is as follows:
[0051] (1) Glyceryl monostearate is heated and melted, and the melting temperature is 90°C.
[0052] (2) In the fluidized bed, adjust an appropriate air volume to fully fluidize the cefuroxime axetil raw material.
[0053] (3) In the fluidized bed, adjust the temperature of the material to 40°C to 50°C, and the atomization pressure to 0.1 to 0.3Mpa. Under the action of compressed air, the glyceryl monostearate is passed through the spray device to form atomized droplets, which are slowly sprayed on the surface of the fully fluidized cefuroxime axetil raw material, so that it is uniformly coated.
[0054] (4) Add sucrose, mannitol, low-substituted hydroxypropyl cellulose and hydroxypropyl cellulose to the above powder, granulate, add the remaining adjuvant after drying, mix and pack, and prepare a dry suspension.
Embodiment 3
[0055] Embodiment 3 (1000 bags of dry suspensions)
[0056]
[0057] The preparation method is as follows:
[0058] (1) Glyceryl monostearate is heated and melted, and the melting temperature is 90°C.
[0059] (2) In the fluidized bed, adjust an appropriate air volume to fully fluidize the cefuroxime axetil raw material.
[0060] (3) In the fluidized bed, adjust the temperature of the material to 40°C to 50°C, and the atomization pressure to 0.1 to 0.3Mpa. Under the action of compressed air, the glyceryl monostearate is passed through the spray device to form atomized droplets, which are slowly sprayed on the surface of the fully fluidized cefuroxime axetil raw material, so that it is uniformly coated.
[0061] (4) Add sucrose, mannitol, low-substituted hydroxypropyl cellulose and hydroxypropyl cellulose to the above powder, granulate, add the remaining excipients after drying, mix and pack separately, and prepare a dry suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com