Crystalline cefuroxime axetil preparation method
A technology for cefuroxime axetil and cefuroxime axetil is applied in the field of chemical synthesis of cefuroxime axetil, can solve the problems of many impurities, safety risks, low boiling point of acetaldehyde, etc., achieves improved catalytic efficiency, improved product quality, simplified effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
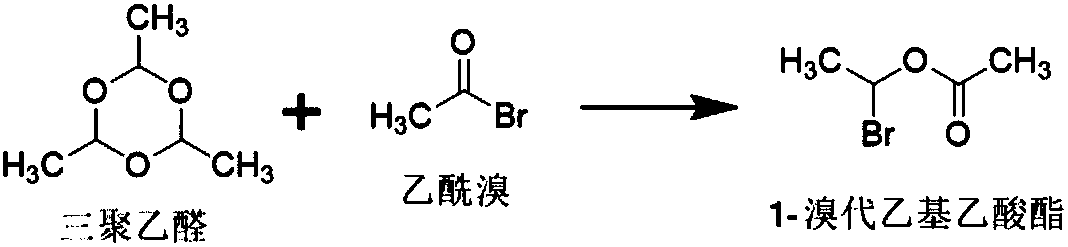
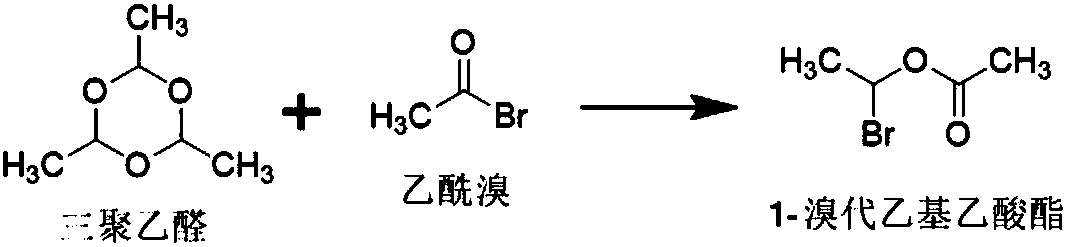
[0049] The present embodiment provides a kind of method for preparing 1-bromoethyl acetate, and described method is specifically:
[0050] In the reaction vessel, add 750.65mmol of acetyl bromide and 0.377mmol of zinc chloride, control the temperature at -5°C to 5°C, dropwise add 238.35mmol of paraldehyde, and maintain the above temperature for 5 hours to obtain crude 1-bromo Ethyl acetate; at a temperature of 0-5°C, wash the crude 1-bromoethyl acetate twice with 1500 mL of purified water, then dry it with 20 g of anhydrous sodium sulfate for 0.5 h, and filter it. That is, 1-bromoethyl acetate.
[0051] The total amount of 1-bromoethyl acetate obtained in this example is 95.6g, the yield: 80.06%, and the GC normalized purity: 97.34%.
Embodiment 2
[0053] The present embodiment provides a kind of method for preparing 1-bromoethyl acetate, and the difference between described method and embodiment 1 is:
[0054] At a temperature of 6-8°C, the crude 1-bromoethyl acetate was washed twice with 1500 mL of purified water, dried with 20 g of anhydrous sodium sulfate for 1 hour, and filtered to obtain 1-bromoethyl acetate ester;
[0055] The total amount of 1-bromoethyl acetate obtained in this example was 94.3g, the yield: 78.97%, and the GC normalized purity: 96.91%.
Embodiment 3
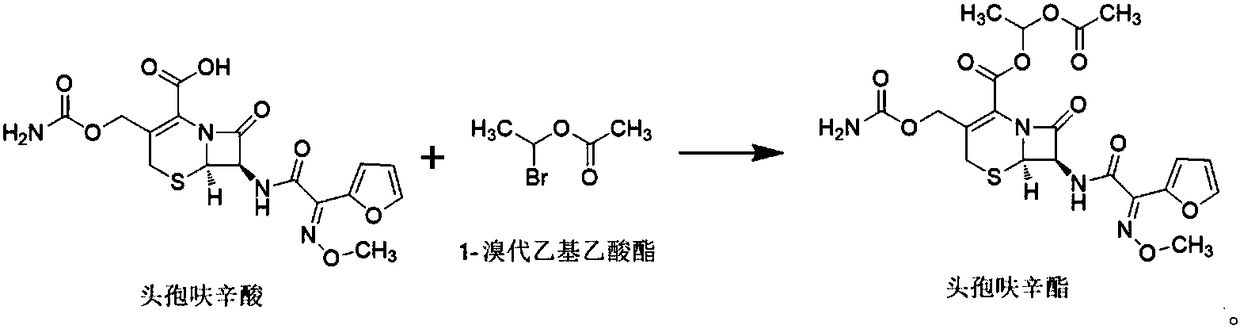
[0057] This embodiment provides a method for preparing cefuroxime axetil using 1-bromoethyl acetate prepared in Example 1, the method being specifically:
[0058] After mixing 400mL of dimethylacetamide and 100mL of purified water, add 0.283mol of cefuroxime acid and 0.192mol of potassium carbonate to it, stir evenly, adjust the temperature to -5°C to 0°C, and then slowly add 0.467mol to it for implementation The 1-bromoethyl acetate obtained in Example 1 was reacted while maintaining the above temperature, and the reaction endpoint was monitored by sampling. When the normalized purity of cefuroxime acid was less than or equal to 2%, it was the reaction endpoint (reaction time was about 1.5h).
[0059] Add 1000mL of ethyl acetate to the reaction solution after the reaction, use saturated sodium bicarbonate solution to adjust its pH value to 7-8, stir it at a speed of 90-150rpm for 1h, and then let it stand for stratification; After washing the organic layer with 500ml of 4% hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com