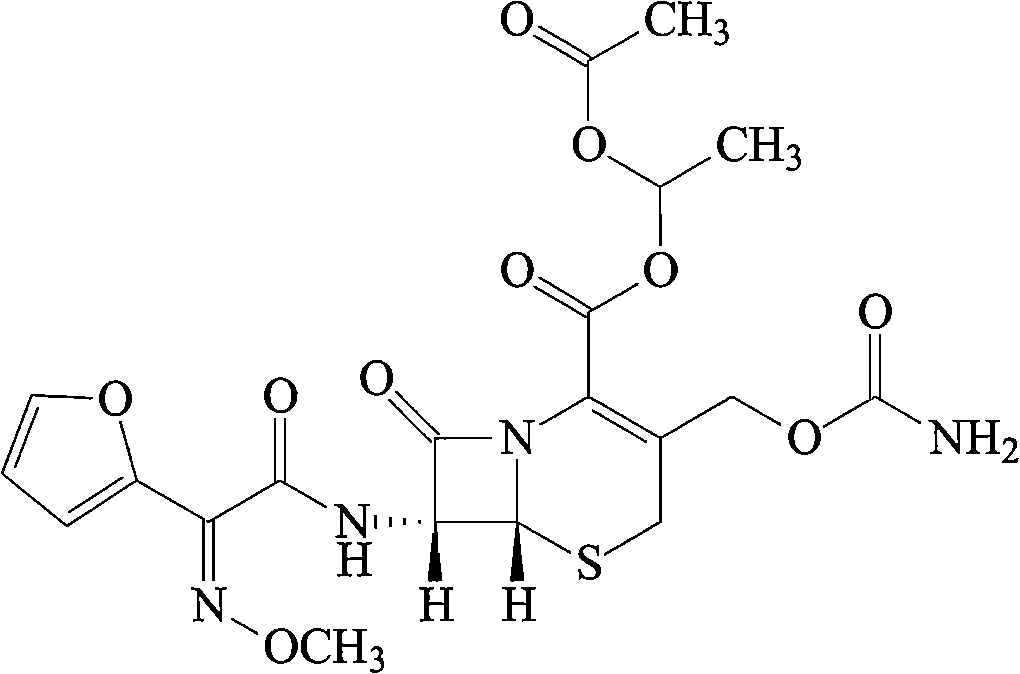
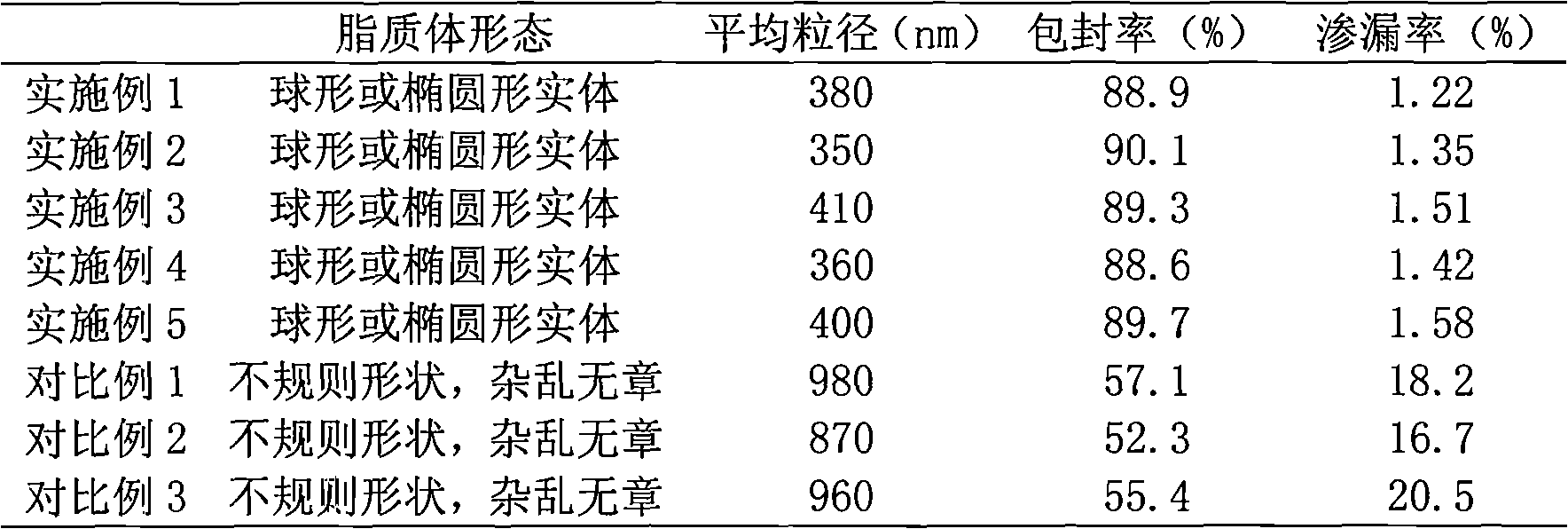
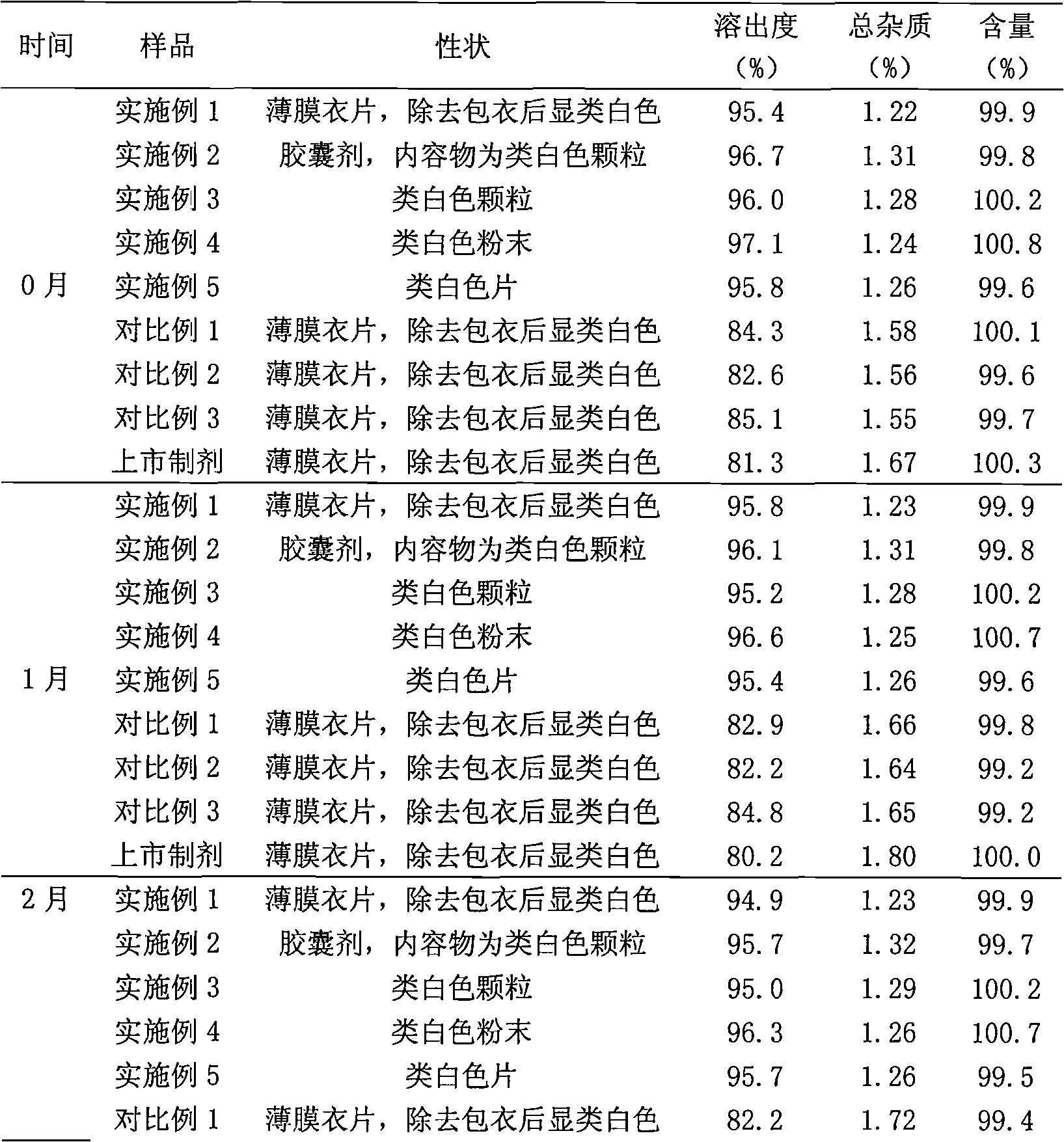
Cefuroxime axetil lipid microsphere solid preparation
The technology of cefuroxime axetil and furoxacil axetil is applied in the field of medicine and can solve the problems of low cefuroxime axetil liposome encapsulation rate, unsatisfactory solubility of cefuroxime axetil, low bioavailability and the like, To achieve the effect of improving drug therapeutic index, increasing quality stability, and reducing drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] On the other hand, the present invention provides the preparation method of above-mentioned cefuroxime axetil lipid microsphere solid preparation, and this method comprises:
[0043] Add gum arabic, sodium deoxycholate and polysorbate 80 into purified water, then add cefuroxime axetil and mix evenly, heat and stir in a water bath at 70-90°C until it melts, use a tissue masher to shear and stir to obtain the primary emulsion , and then cyclically emulsified by a high-pressure homogenizer to obtain an emulsion, and then freeze-dried or spray-dried to obtain lipid microspheres.
[0044] In a preferred embodiment of the preparation method of the cefuroxime axetil lipid microsphere solid preparation of the present invention, the tissue masher used is a JJ-2B type high-speed tissue masher, and the rotating speed for shearing is 12000-15000r / min, The time for shearing and stirring is 10-20 minutes.
[0045] Among them, as a high-pressure milk homogenizer, the model NS1001L im...
Embodiment 1
[0070] The preparation of embodiment 1 cefuroxime axetil lipid microsphere tablet
[0071] The raw and auxiliary materials used are as follows:
[0072] Cefuroxime Axetil 125g
[0073] Gum Arabic 500g
[0074] Sodium deoxycholate 225g
[0075] Polysorbate 80 312.5g
[0076] Starch 800g
[0077] Microcrystalline Cellulose 200g
[0078] Croscarmellose Sodium 200g
[0079] Povidone K30 50g
[0081] Preparation Process:
[0082] (1) Add 500g of gum arabic, 225g of sodium deoxycholate and 312.5g of polysorbate 80 into 2000ml of purified water, then add 125g of cefuroxime axetil and mix evenly, heat and stir in a water bath at 90°C until it melts, and use a tissue masher Shearing and stirring for 20 minutes at a rotational speed of 12000r / min to obtain the first emulsion, and then cyclically emulsifying it through a high-pressure homogenizer for 5 times to obtain an emulsion, and then freeze-drying to obtain lipid microspheres.
[0083] (2) ...
Embodiment 2
[0084] Example 2 Preparation of Cefuroxime Axetil Lipid Microsphere Capsules
[0085] The raw and auxiliary materials used are as follows:
[0086] Cefuroxime Axetil 125g
[0087] Gum Arabic 875g
[0088] Sodium deoxycholate 625g
[0089] Polysorbate 80 375g
[0090] Microcrystalline Cellulose 1000g
[0091] Carboxymethyl Starch Sodium 500g
[0092] Povidone K30 20g
[0094] Preparation Process:
[0095](1) Add 875g of gum arabic, 625g of sodium deoxycholate and 375g of polysorbate 80 into 9000ml of purified water, then add 125g of cefuroxime axetil and mix evenly, heat and stir in a water bath at 70°C until it melts, and cut it with a tissue masher. Cut and stir for 10 minutes at a rotational speed of 15000r / min to obtain the primary emulsion, and then circulate and emulsify it through a high-pressure homogenizer for 5 times to obtain an emulsion, which is then spray-dried to obtain lipid microspheres.
[0096] (2) Mix the prepared lipid mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com