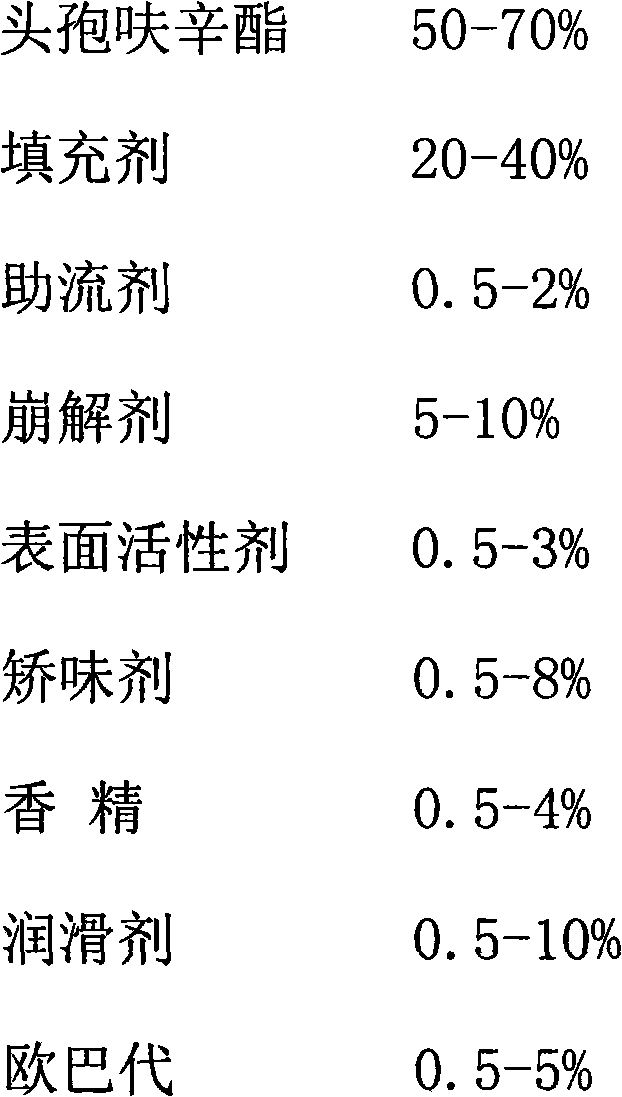
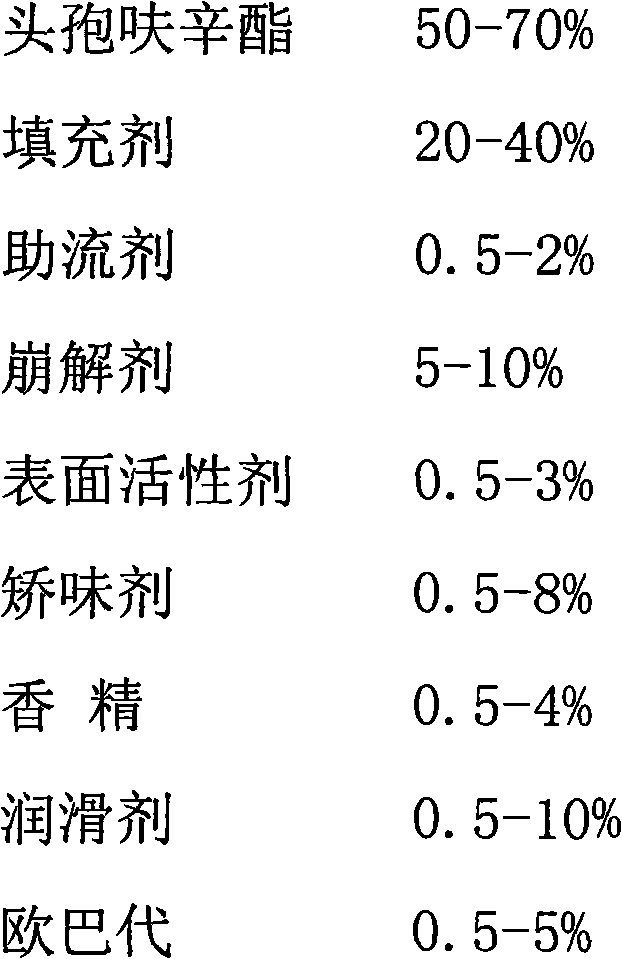
Dispersible tablet of cefuroxime axetil
A technology of cefuroxime axetil and dispersible tablets, which can be used in pill delivery, pharmaceutical formulations, antibacterial drugs, etc. It can solve the problems of increasing the cost of excipients, increasing the amount of excipients, and discoloration of raw materials, so as to improve bioavailability and reduce dispersion. time, effect of improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation:
[0025] Mix cefuroxime axetil, microcrystalline cellulose, crospovidone, sodium lauryl sulfate, colloidal silicon dioxide, orange powder flavor, aspartame, part of stearic acid, and dry granulation to make In the granulation process, 30 meshes are used for whole granulation. During the dry cycle granulation process, the proportion of particles between 30 mesh and 60 mesh sieve is more than 40% as the end point. After the tablet is compressed, the plain tablets are coated according to the conventional film coating operation. When the weight gain of the tablets reaches about 1.5%, the spraying is stopped, and the air is dried to make 1000 tablets.
[0026] The dissolution of cefuroxime axetil dispersible tablets was tested in accordance with the method under the "Chinese Pharmacopoeia" 2010 edition of cefuroxime axetil tablets. The test result reached 97.1% in 15 minutes, the content was 98.2%, and the uniformity of dispersion was dispersed in 1 minute. , Sweet...
Embodiment 2
[0029] Preparation method: same as Example 1, to prepare 1000 tablets.
[0030] The dissolution rate of cefuroxime axetil dispersible tablets was tested according to the method under "Chinese Pharmacopoeia" 2010 edition cefuroxime axetil tablets. The test result reached 98.1% in 15 minutes, and the content was 99.2%. The uniformity of dispersion has been dispersed in 1 minute. , Sweet. Placed for 6 months under the conditions of temperature 40℃±2℃ and humidity 75%±5%, the dissolution rate is 98.5% in 15 minutes, the content is 98.8%, and the uniformity of dispersion is 1 minute and 30 seconds. sweet.
Embodiment 3
5g
[0034] Preparation method: same as Example 1, to prepare 1000 tablets.
[0035] The dissolution of cefuroxime axetil dispersible tablets was tested in accordance with the method under the "Chinese Pharmacopoeia" 2010 edition of cefuroxime axetil tablets. The test result reached 99.1% in 15 minutes, and the content was 99.2%. The uniformity of dispersion was dispersed in 1 minute. , Sweet. Placed for 6 months under the temperature of 40℃±2℃ and humidity of 75%±5%, the dissolution rate is 97.2% for 15 minutes, the content is 99.2%, and the uniformity of dispersion is 1 minute and 30 seconds. sweet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com