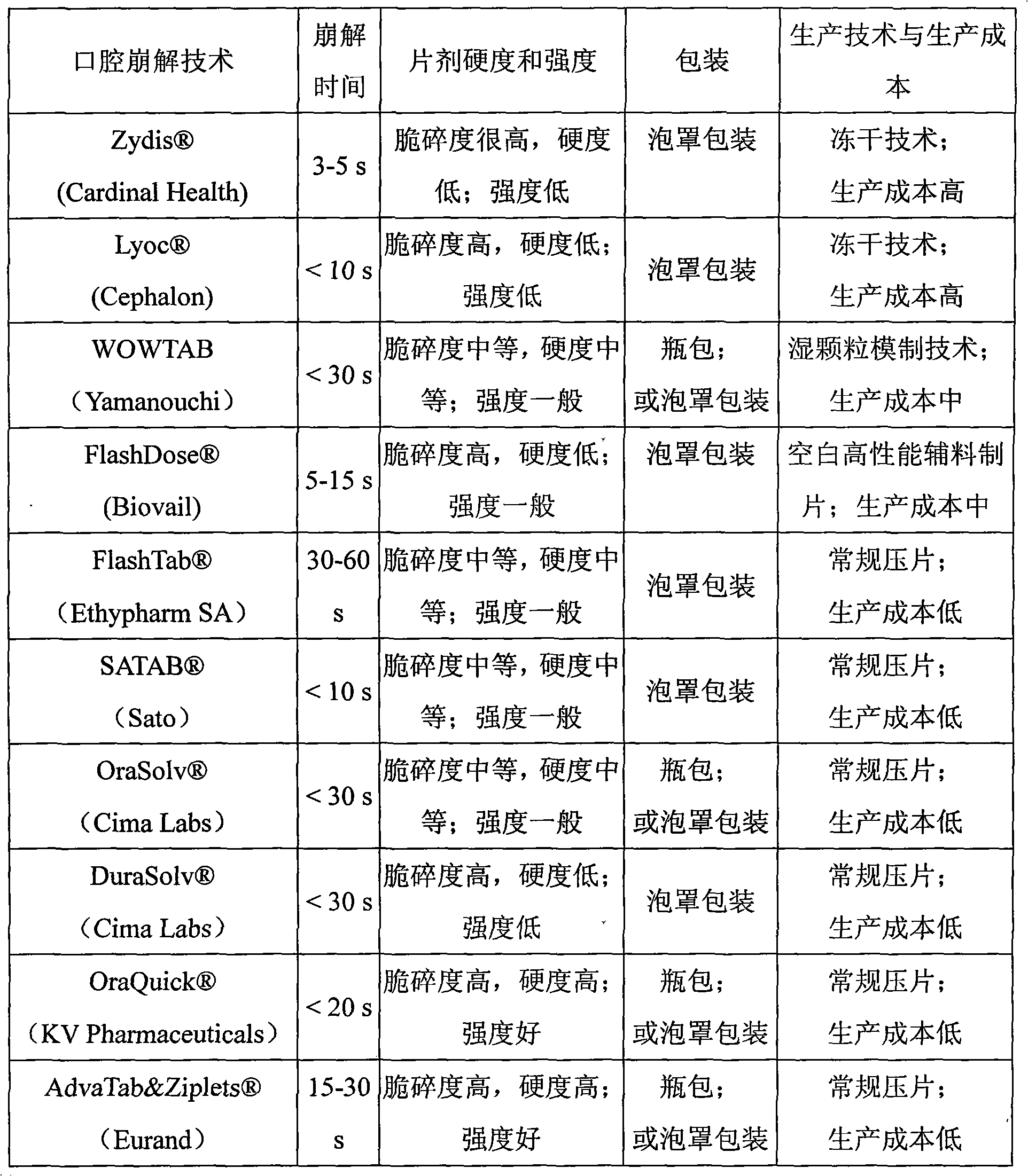
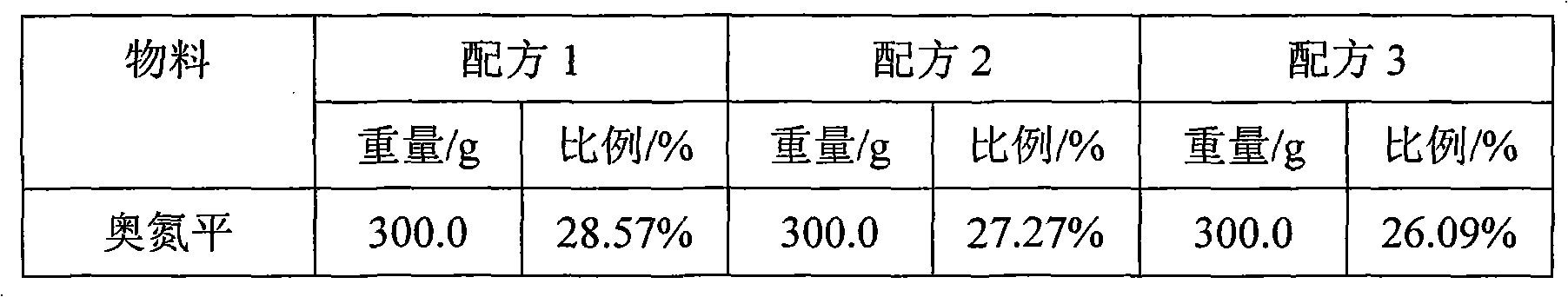
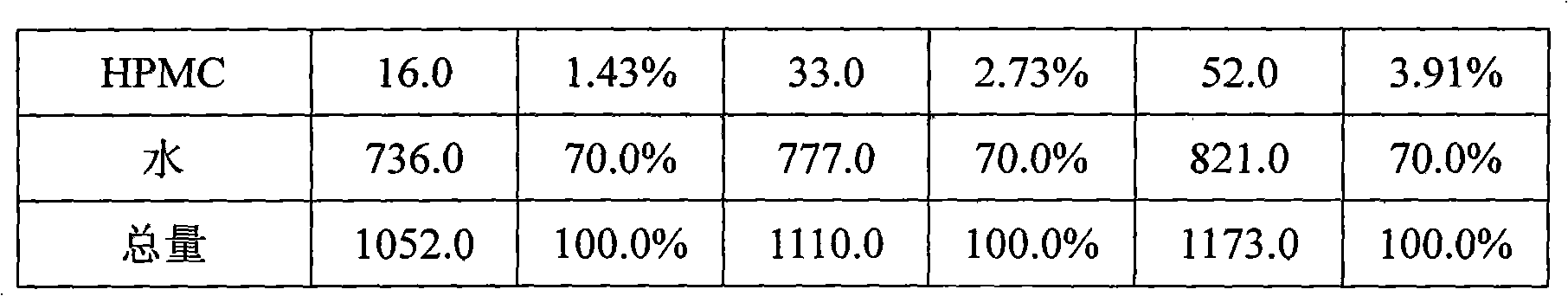
Novel Olanzapine orally disintegrating tablet
An orally disintegrating tablet and olanzapine technology are applied in the field of preparation of the olanzapine orally disintegrating tablet, and can solve the problems of non-compliance, negative influence of treatment effect, poor treatment effect, etc., so as to cover bitter taste and improve treatment compliance. sex, improve taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] (1) Production of olanzapine coated particles
[0169] In a fluidized bed coating granulator [produced by Chongqing Yingge (China), WBF-III type], load 1500 g of spherical granulated products (microcrystalline cellulose) with an average particle size of 106-212 μm and containing microcrystalline cellulose Cellulose CP 102 is manufactured by Asahi Chemical Co., Ltd (Japan). The air inlet temperature and material temperature are controlled at 50-70°C and 35-45°C respectively, and the atomization pressure is controlled at 1.5-2.5Bar. The olanzapine coating disperse phase is coated on the neutral core with the spray rate of 16g / min by spray coating process.When the olanzapine coating disperse phase of prescribed amount has been coated, stop this bottom spray coating operation, The resulting granules were then dried in a coating granulator to obtain approximately 1807 g of olanzapine coated granules.
[0170] Olanzapine coating phase:
[0171] Olanzapine (form II, particle...
Embodiment 2
[0209] (1) Production of olanzapine coated particles
[0210] In a fluidized bed coating granulator [produced by Chongqing Yingge (China), WBF-III type], load 1500g average particle diameter about 100 μm spray-dried mannitol spherical granules (mannitol 100SD, produced by Roquette (France) product) production. Control the air inlet temperature and material temperature at 50-70°C and about 35-45°C respectively, control the atomization pressure at 1.5-2.5Bar, and apply olanzapine at a spray rate of 20g / min by top spray coating process The dispersed phase is coated on a neutral core. The top spray coating operation was stopped when a prescribed amount of olanzapine-coated dispersed phase had been coated, and then the resulting granules were dried in a coating granulator to obtain about 2000 g of olanzapine-coated granules.
[0211] Olanzapine coating phase:
[0212] Olanzapine (form II, particle size D(V, 0.9)≤20μm) 500g
[0213] HPMC E5 PREM LV 85g
[0214] Purified water 1...
Embodiment 3
[0229] (1) Production of olanzapine coated particles
[0230] In a fluidized bed coating granulator [produced by Chongqing Yingge (China), WBF-III type], load 1200g average particle diameter about 100 μ m spray-dried mannitol spherical particles (mannitol 100SD, produced by Roquette (France) product) production. Control the air inlet temperature and material temperature at 50-70°C and about 35-45°C respectively, control the atomization pressure at 1.5-2.5Bar, and apply olanzapine at a spray rate of 19g / min by top spray coating process The dispersed phase is coated on a neutral core. The top spray coating operation was stopped when the prescribed amount of the olanzapine-coated dispersed phase had been coated, and then the resulting granules were dried in a coating granulator to obtain about 1300 g of olanzapine-coated granules.
[0231] Olanzapine coating phase:
[0232] Olanzapine (form II, particle size D(V, 0.9)≤20μm) 150g
[0233] PVP K30 25g
[0234] Purified water 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com