Method for preparing norfloxacin capsule
The technology of norfloxacin and capsules is applied in the field of preparation of norfloxacin capsules, which can solve the problems of low oral bioavailability, insoluble norfloxacin in water, unstable properties, etc. Enhanced stability and fast release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
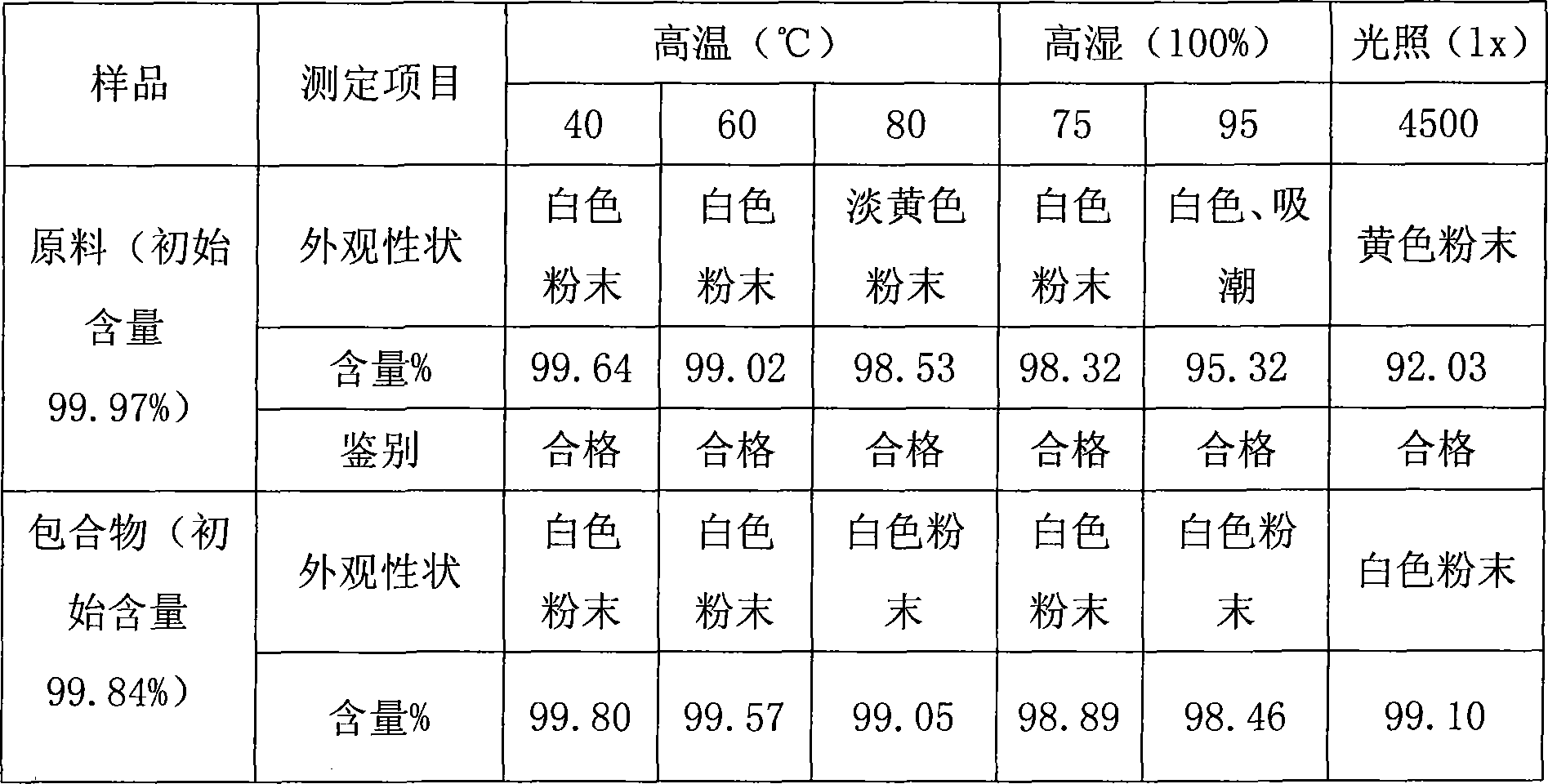
[0017] ①Take norfloxacin and β-cyclodextrin (norfloxacin: β-cyclodextrin = 1:1mol / mol) in a mortar and mix well, then add water to grind into a paste, and keep grinding For 6 hours, the temperature was controlled at 30°C until the inclusion complex of norfloxacin β-cyclodextrin was formed. ② Spread the clathrate flat, and bake it in an oven at 60°C for 7-8 hours until it is completely dry. ③ pulverize the dried medicine, pass through an 80-mesh sieve to granulate, and dry at 60°C until the water content is below 9%. ④ Determine the content of norfloxacin, fill it into No. 0 empty capsules after conversion, so that each capsule contains 0.1 g of norfloxacin, and finally pack it.
[0018] The inclusion rate is 70%. The cumulative dissolution rate within 5 minutes was 99.3%.
Embodiment 2
[0020] ①Take norfloxacin and β-cyclodextrin (norfloxacin: β-cyclodextrin = 1:1mol / mol) in a mortar and mix well, then add water to grind into a paste, and keep grinding For 10 hours, the temperature was controlled at 20-25°C until the inclusion complex of norfloxacin β-cyclodextrin was formed. ② Spread the clathrate flat, place it in a decompression drying oven at 50°C for vacuum drying, and bake for 3 to 4 hours until it is completely dry. ③ pulverize the dried medicine, pass through an 80-mesh sieve to granulate, and place in a decompression drying oven at a temperature of 50° C. under vacuum (2 × 10 4 ~3×10 4 Pa), dried to below 9% moisture. ④ Determine the content of norfloxacin, fill it into No. 0 empty capsules after conversion, so that each capsule contains 0.1 g of norfloxacin, and finally pack it.
[0021] The inclusion rate is 84%. The cumulative dissolution rate within 5 minutes was 99.5%.
Embodiment 3
[0023] ①Take norfloxacin and β-cyclodextrin (norfloxacin: β-cyclodextrin = 1:1mol / mol) in a mortar and mix well, then add water to grind into a paste, and keep grinding For 10 hours, the temperature was controlled at 30° C. until the inclusion complex of norfloxacin β-cyclodextrin was formed. ② Spread the clathrate flat, and place it in an oven at 60°C to dry completely. ③ pulverize the dried medicine, pass through an 80-mesh sieve to granulate, and dry at 60°C until the water content is below 9%. ④ Determine the content of norfloxacin, fill it into No. 0 empty capsules after conversion, so that each capsule contains 0.1 g of norfloxacin, and finally pack it.
[0024] The inclusion rate is 74%. The cumulative dissolution rate within 5 minutes was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com