Method for detecting quality of senile coughand asthmatablets
A quality inspection method, the technology of Kechuan Tablets, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of complex operation, inability to scientifically control the quality of the Elderly Kechuan Tablets, and the inability to qualitatively identify the Elderly Kechuan Tablets.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
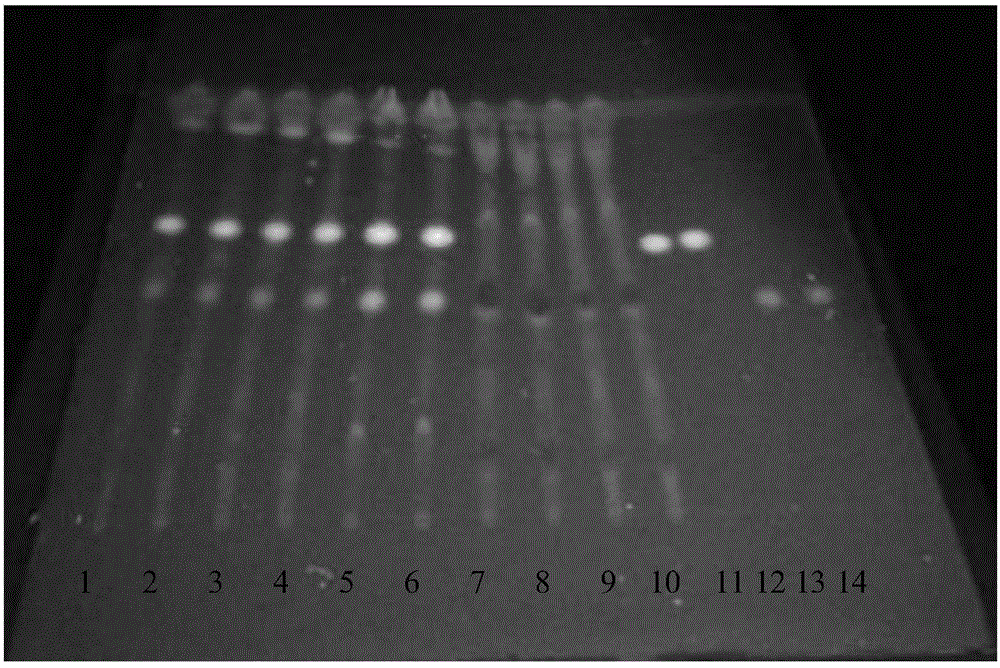
[0098] Example 1 Identify the active ingredients in the Old Kechuan Tablets according to the following method
[0099] A. Identification of the active ingredient Fangfeng:
[0100] S1. Preparation of the test solution: take 3 g of the old cough and asthma tablet powder with the film coat removed, grind it finely, add 20 mL of acetone, ultrasonicate for 30 minutes, filter, evaporate the filtrate to dryness, add 1 mL of ethanol to the residue to dissolve, and use it as the test product product solution;
[0101] S2. Preparation of contrasting medicinal material solution: get 1g of Radix Fangfeng contrasting medicinal material, make contrasting medicinal material solution with the method for need testing solution;
[0102] S3. Preparation of reference substance solution: take acteoside reference substance and 5-O-methylvisamigoside reference substance, add methanol to make a mixed solution containing 1 mg per 1 mL, as the reference substance solution;
[0103] S4. Preparation o...
Embodiment 2
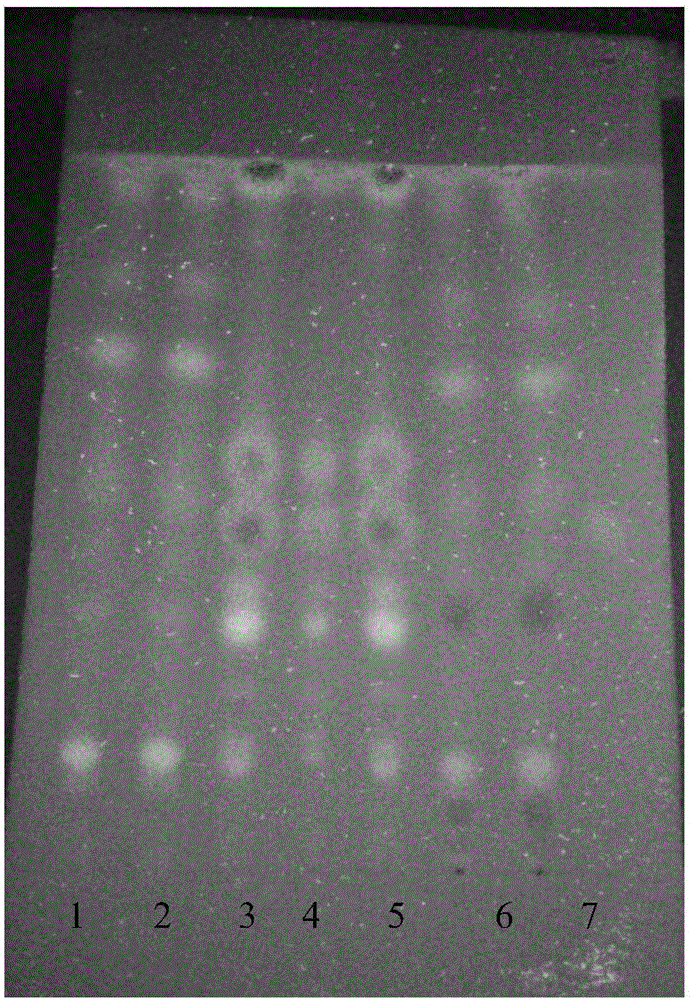
[0133] Example 2 Identify the active ingredients in the Old Kechuan Tablets according to the following method
[0134] A. Identification of the active ingredient Fangfeng:
[0135] S1. Preparation of the test solution: take 1 g of the old cough and asthma tablet powder with the film coat removed, grind it finely, add 10 mL of acetone, ultrasonicate for 10 minutes, filter, evaporate the filtrate to dryness, add 2 mL of ethanol to the residue to dissolve, and use it as the test product product solution;
[0136] S2. the preparation of contrast medicinal material solution: get Radix Fangfeng contrast medicinal material 2g, make contrast medicinal material solution with the method for need testing solution;
[0137] S3. Preparation of reference substance solution: take acteoside reference substance and 5-O-methylvisamigoside reference substance, add methanol to make a mixed solution containing 1 mg per 1 mL, as the reference substance solution;
[0138] S4. Preparation of negati...
Embodiment 3
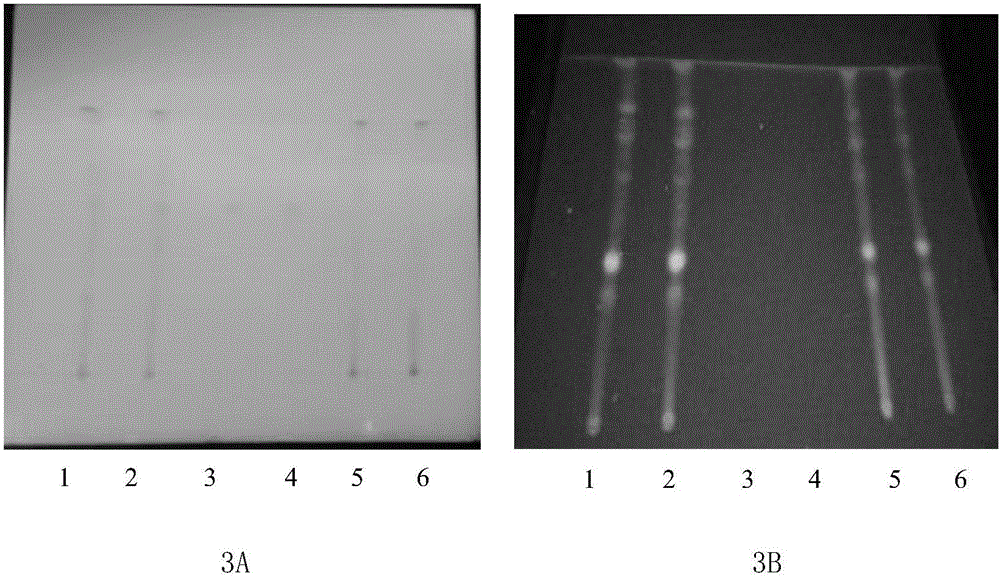
[0167] Example 3 According to the following method, the active ingredients in the Old Kechuan Tablets are identified
[0168] A. Identification of the active ingredient Fangfeng:
[0169] S1. Preparation of the test solution: take 6 g of old cough and asthma tablet powder with film coating removed, grind it finely, add 40 mL of acetone, ultrasonicate for 50 minutes, filter, evaporate the filtrate to dryness, add 3 mL of ethanol to the residue to dissolve, and use as test product solution;
[0170] S2. the preparation of contrast medicinal material solution: get the windproof contrast medicinal material 3g, make contrast medicinal material solution with the method for need testing solution;
[0171] S3. Preparation of reference substance solution: take acteoside reference substance and 5-O-methylvisamigoside reference substance, add methanol to make a mixed solution containing 1 mg per 1 mL, as the reference substance solution;
[0172] S4. Preparation of Negative Control Sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com