Method for separating and detecting folic acid and folic acid optical isomers in folic acid
A technology of optical isomers and folic acid, applied in the field of drug analysis, can solve the problems of difficult separation and drug analysis obstacles, and achieve the effect of simple method and fast peak time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Main instruments: Shimadzu LC-20A high performance liquid chromatography.
[0056] mobile phase:
[0057] Mobile phase A: methanol: 40-60, acetonitrile: 40-60;
[0058] Mobile phase B: acetic acid: 2-4, triethylamine: 2-4;
[0059] Methanol-acetonitrile-acetic acid-triethylamine (40~60:40~60:2~4:2~4), the best effect group is 50:50:3:3;
[0060] Chromatographic column: O-9-(tert-butylcarbamoyl)quinine covalently bonded to the surface of silica gel as filler, 4.6mm×150mm, 5μm;
[0061] Flow rate: 0.4~0.6ml / min;
[0062] Detection wavelength: 280±10nm;
[0063] Column temperature: 20-30°C, preferably 25°C;
[0064] Diluent: tetrahydrofuran - methanol - water - acetic acid - triethylamine (10:80:10:2.4:3);
[0065] Injection volume: 5μl;
[0066] The test solution: 0.3mg / ml;
[0067] Quantitative method: peak area normalization method.
Embodiment 2
[0069] Solution preparation:
[0070] Avoid light operation.
[0071] The test solution: take an appropriate amount of this product (folic acid), add diluent [tetrahydrofuran-methanol-water-acetic acid-triethylamine (10:80:10:2.4:3)] ultrasonically dissolve and quantitatively dilute to make 1ml Contain about 0.3mg of the solution, shake well, that is.
[0072] System suitability solution: Take an appropriate amount of folic acid optical isomer system suitability reference substance (containing folic acid and optical isomer), add diluent to ultrasonically dissolve and dilute to make a solution containing about 0.3mg per 1ml, shake well, that is have to.
Embodiment 3
[0074] testing method
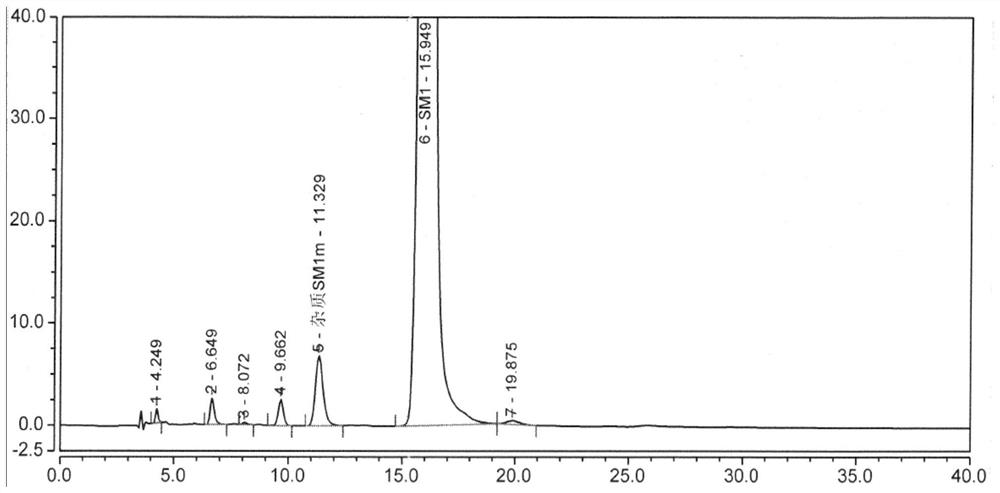
[0075] Precisely measure 5 μl of the system suitability solution, inject it into the liquid chromatograph, and record the chromatogram. figure 1 ).
[0076] Table 1 Integral results of system suitability testing
[0077]
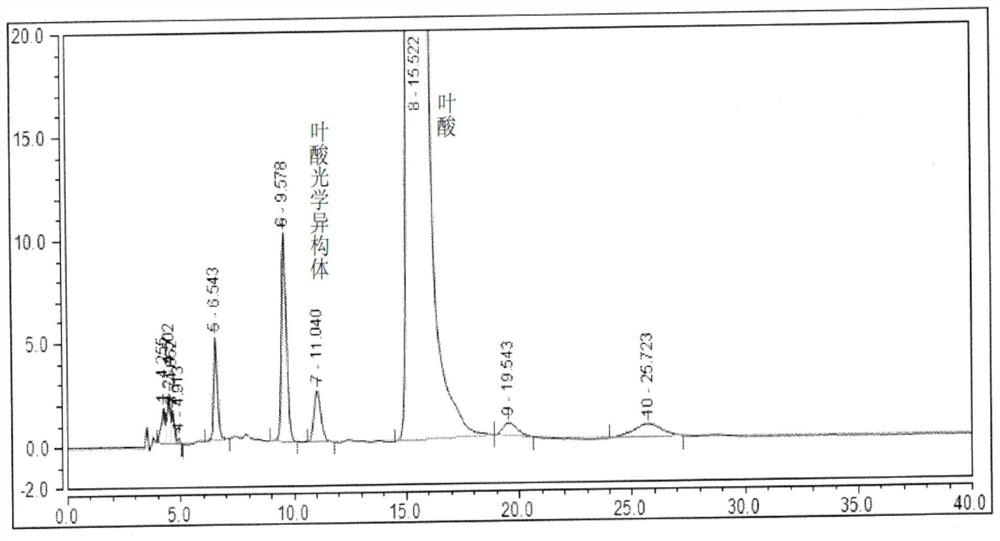
[0078] All impurities of folic acid are mixed, including impurities A, B, C, D, E, F, G, H and SM1d, SM1k and folic acid optical isomer impurities in EP standard (attached figure 2 ).
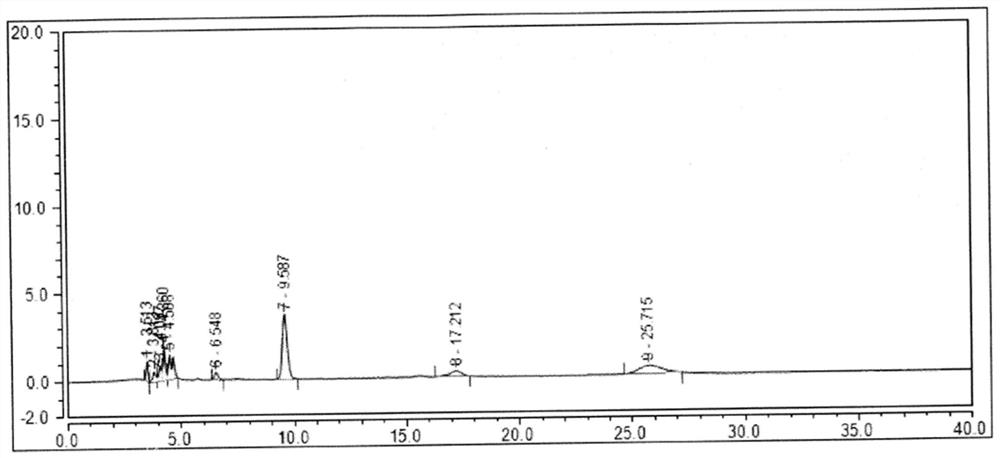
[0079] Other impurities in folic acid are mixed, including EP impurities A, B, C, D, E, F, G, H and SM1d, SM1k (attached image 3 ).
[0080] Precisely measure 5 μ l of the test solution, inject it into a liquid chromatograph, record the chromatogram to 2.5 times the retention time of the main peak, and calculate the content of the optical isomers by the peak area normalization method for the two peaks of the optical isomers and folic acid. The content of isomers does not exceed 0.15%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com