Content determination method of sanguisorbin I
A technology of Burnet saponins and a determination method, which are applied in the directions of measuring devices, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of long peak time, inability to effectively separate saponins, and low analysis efficiency, and achieve The effect of short peak time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Selection of high performance liquid chromatography column, mobile phase and elution program
[0069] Sanyu saponins belong to triterpene saponins, which have relatively high polarity. Experiments show that they are retained on C18 chromatographic columns, so C18 analytical columns are used.
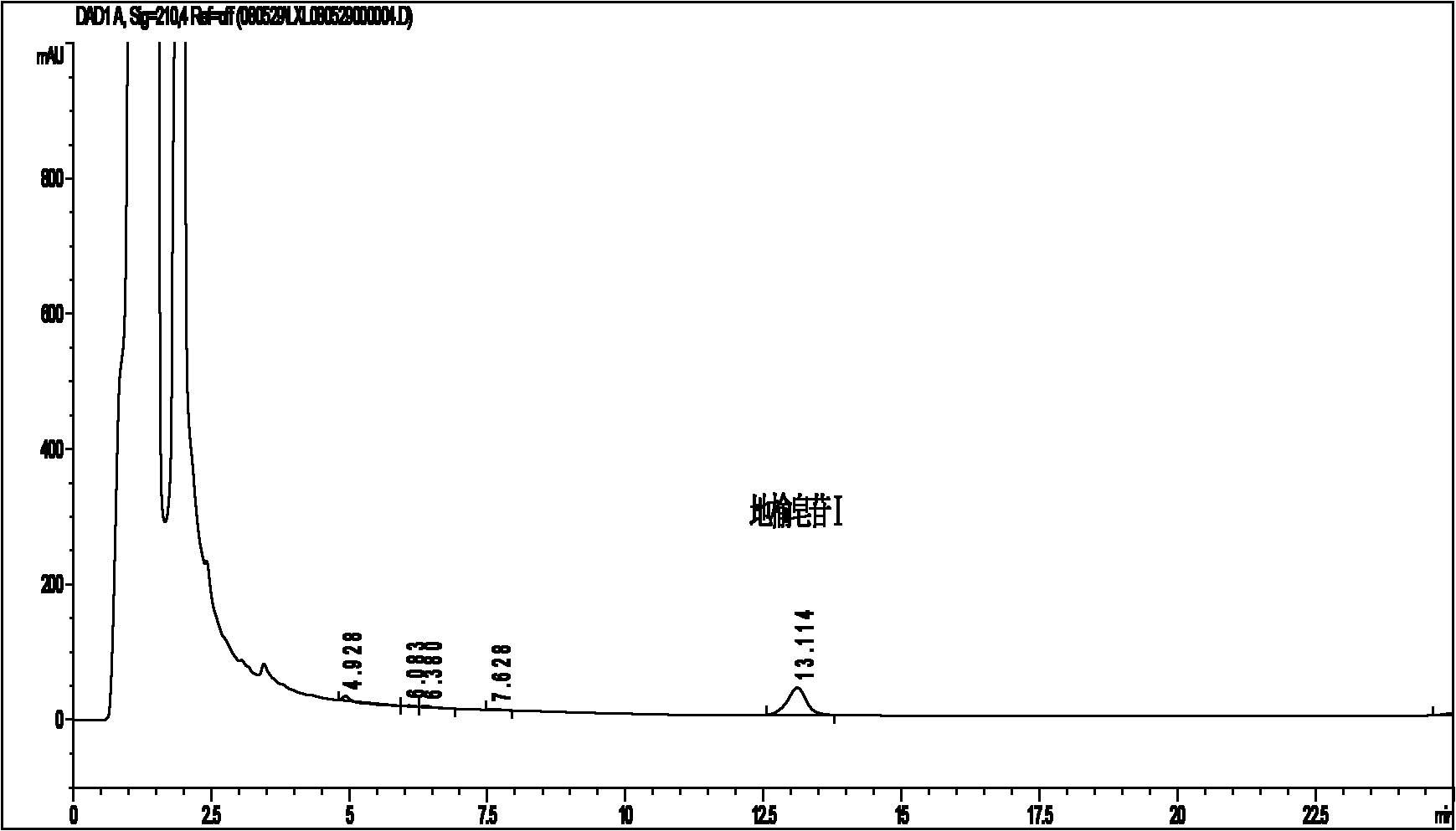
[0070] The choice of mobile phase: Sanguirin I is a neutral compound, and there is no need to add acid-base additives or ion pair reagents in the mobile phase. Therefore, the mobile phase systems such as methanol-water and acetonitrile-water are selected for comparison and screening. In UV detection, the acetonitrile-water system has a straighter baseline and sharper peaks than the methanol-water system. However, after multiple adjustments, the separation chromatographic peak is still better than methanol-water. There are few systems, and the impurity peak immediately before the saponin I can not be detected at all whether using isocratic elution or gradient elution (see Figu...
Embodiment 2
[0076] Example 2 The effect of column particle size on the separation effect of Sanguis saponins I reference substance
[0077] (1) Sample solution preparation: Take an appropriate amount of the crude Sanguisaponin I, dissolve it with methanol and dilute it to a solution containing 0.5mg per 1ml to obtain;
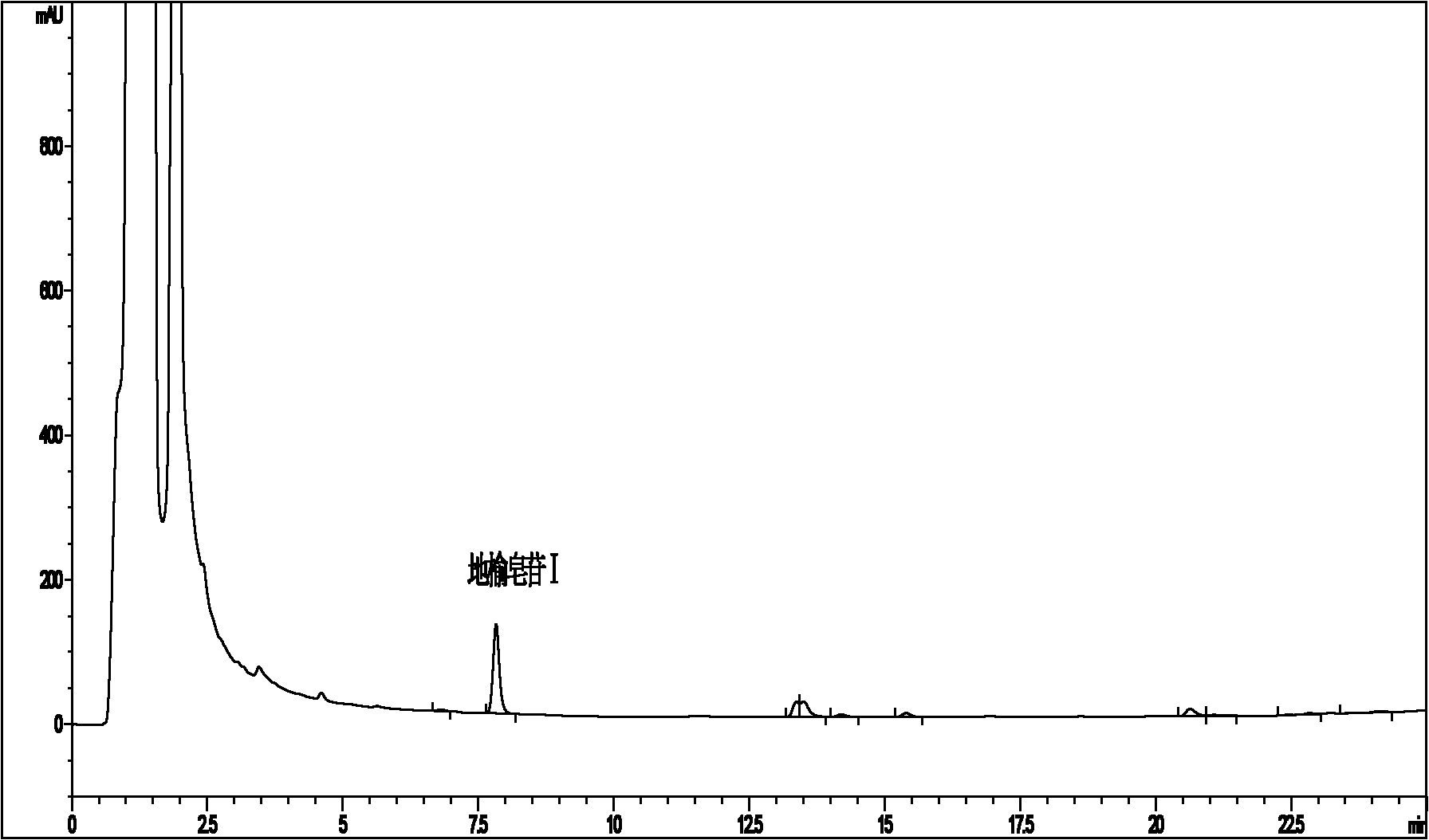
[0078] (2) Chromatographic conditions: use methanol-water system as mobile phase gradient elution, the elution procedure is 0~7min 60%→64% methanol aqueous solution, 7~12min 64% methanol aqueous solution, 12~20min 64%→80 % Methanol aqueous solution, 20~27min 80% methanol aqueous solution, flow rate 1.0ml / min, column temperature 25℃, detection wavelength 210nm, ELSD: 85℃, 2.8L / min, gain1; YMC-Pack proC was investigated 18 Column (150×4.6mm, 3.5μm) (UV detection) and SynergiHydro-RP C 18 The separation effect of the column (150×4.6mm, 4μm) (ELSD detection) on Sanguisorba saponins I is shown in Figure 2.
[0079] The results in Fig. 2 show that the main peak of Sanguirin I and the i...
Embodiment 3
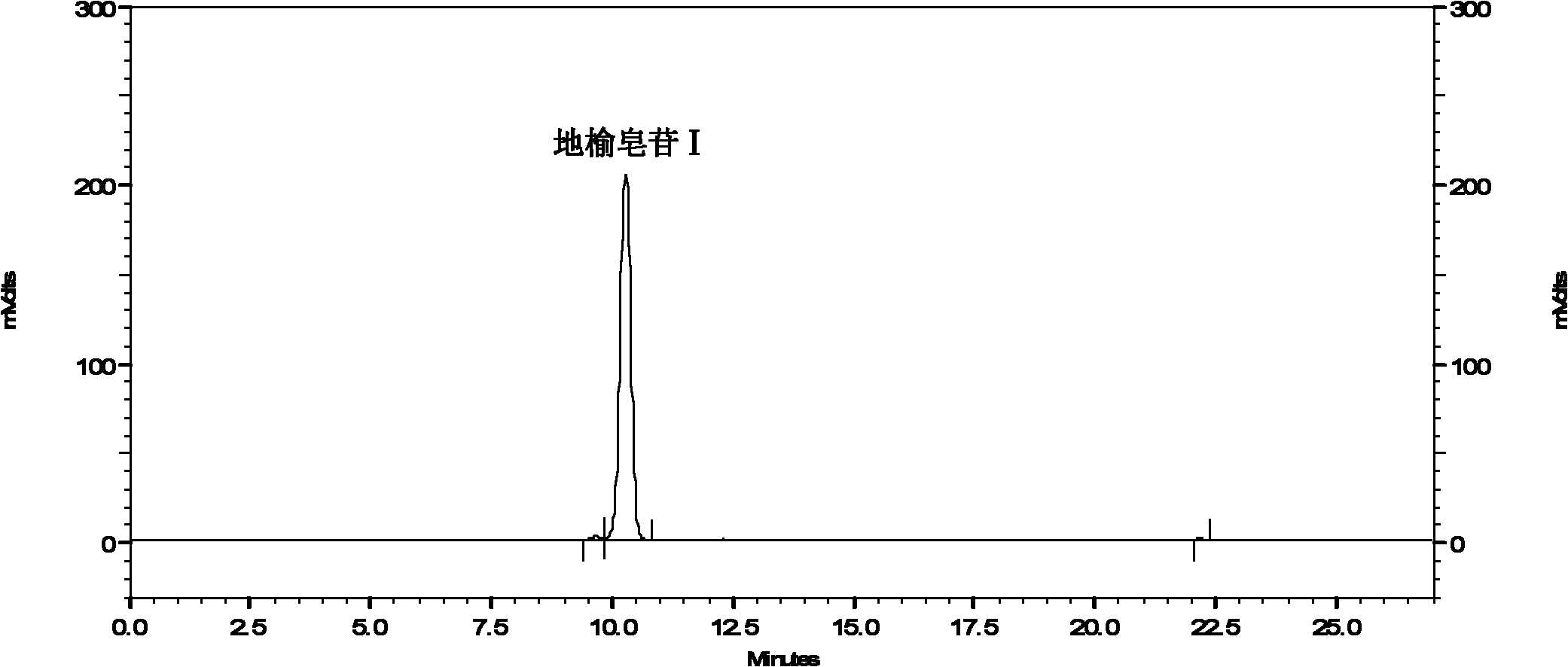
[0080] Example 3 Isocratic elution of methanol-water system for separation of Sanguisorba saponins I from Sanguisorba sanguis medicinal materials and total Sanguis saponins
[0081] (1) Preparation of medicinal material sample solution: Take about 0.4g of Sanguiran medicinal material powder (passed through No. 4 sieve), accurately weigh it, place it in a 100ml measuring flask, add 80ml of 60% ethanol aqueous solution, shake well, ultrasound 15min, and place until Set the volume to the mark at room temperature, shake well, filter and take the filtrate for sample analysis or centrifuge to get the supernatant.
[0082] (2) Preparation of the sample solution of the total glycosides of Sanguinis: take an appropriate amount of the crude powder of Sanguirell medicinal materials, add 10 times the volume of water to decoct, each time for 1 hour, cook three times, filter, combine the filtrate, apply the macroporous adsorption resin, and wash thoroughly with distilled water , Then eluted with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com