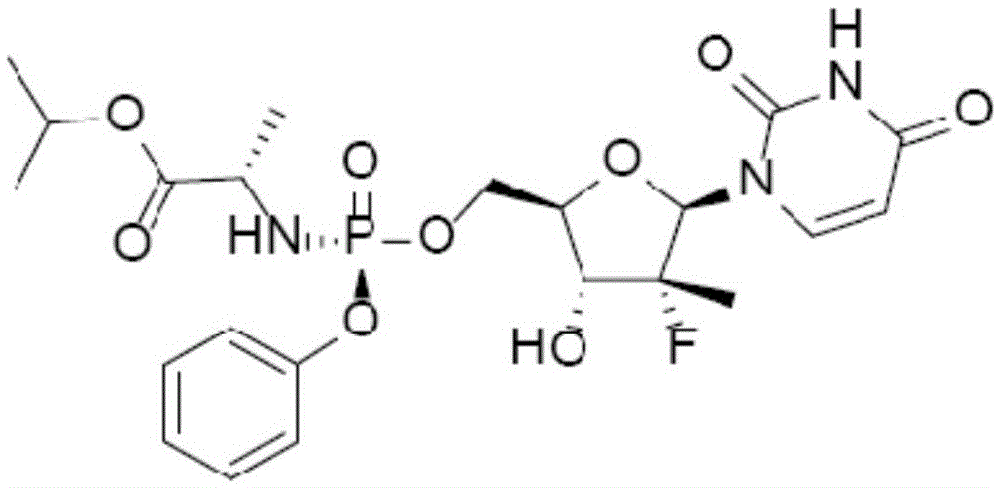
Sofosbuvir dispersible tablet and preparation method thereof
A technology of dispersible tablets and mass percentage, applied in the field of medicine, can solve the problems of large volume, inconvenient taking, difficult treatment and the like, and achieve the effects of simple preparation process, convenient taking and high bioavailability
Inactive Publication Date: 2017-06-23
PEKING UNIV FOUNDER GRP CO LTD +2
View PDF12 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Ordinary tablets are large in size, or often need to use multiple tablets (grains) at a time, and need to be taken with water, which is inconvenient to take, especially for the elderly, young and patients with swallowing dysfunction.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
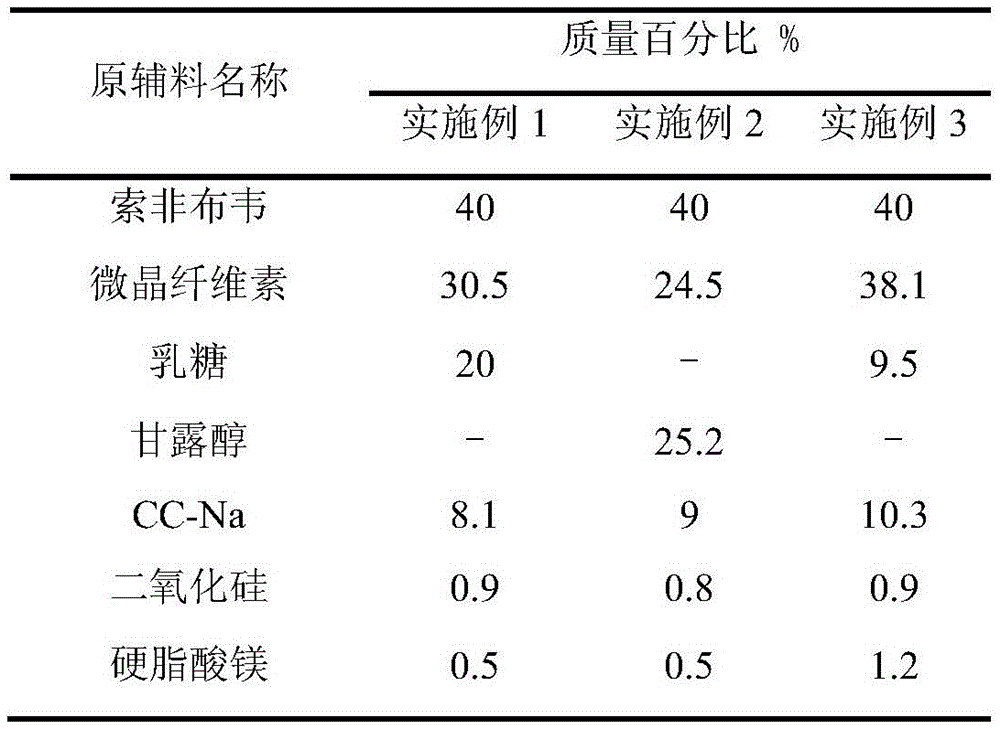
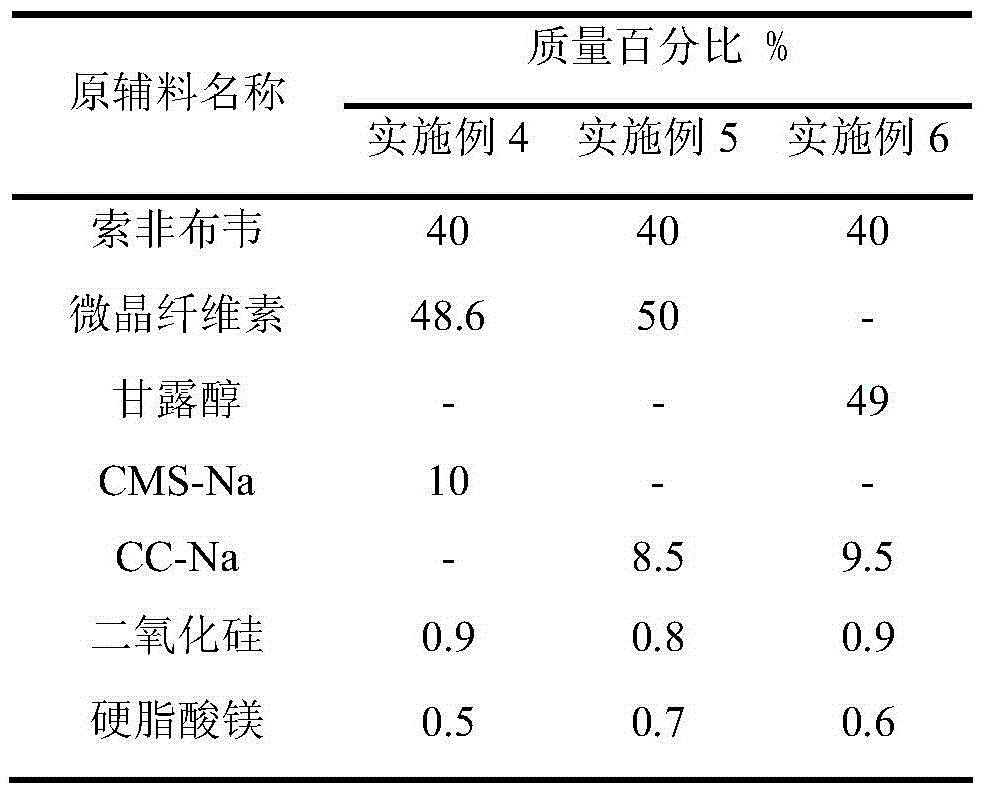
Embodiment 1
[0044] Example 1 sample: 1001 mg / tablet, 6 tablets;
Embodiment 2
[0045] Example 2 sample: 1002 mg / tablet, 6 tablets;
Embodiment 3
[0046] Example 3 sample: 1001 mg / tablet, 6 tablets;
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a sofosbuvir dispersible tablet and a preparation method thereof. The dispersible tablet consists of the following components in percentage by mass: 30-70% of sofosbuvir, which serves as a raw material, and 40-90% of adjuvants, wherein the adjuvants include a filler, a disintegrating agent and a lubricating agent; and the preparation method comprises processes of weighing the raw material and the adjuvants, mixing the raw material and the adjuvants and tableting an obtained mixture. The dispersible tablet prepared by the invention can be completely disintegrated rapidly, so that a time to peak is shortened and drug absorbency; the dispersible tablet has the characteristics of being convenient to take, rapid in efficacy release, high in bioavailability and the like; and the preparation method is simple in process, easy for control, convenient to operate and suitable for industrial production.
Description
technical field [0001] The invention belongs to the field of medicines, and in particular relates to a Sofosbuvir dispersible tablet and a preparation method thereof. Background technique [0002] Hepatitis C virus hepatitis C or hepatitis C for short, is a kind of viral hepatitis caused by hepatitis C virus (Hepatitis C Virus, HCV) infection, a group of systemic infectious diseases mainly based on liver damage, its dosage form Symptoms are not obvious during the infection period and tend to become chronic. Hepatitis C can lead to chronic inflammation, necrosis and fibrosis of the liver, and some patients can develop liver cirrhosis or even hepatocellular carcinoma. The successful listing of sofosbuvir, when used in the treatment of specific genotypes of chronic hepatitis C, can eliminate the need for traditional injections of interferon (IFN), reduce adverse reactions and improve patient compliance, and are widely considered It is a breakthrough drug for chronic hepatitis...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/20A61K31/7072A61K47/38A61P31/14A61P1/16
CPCA61K9/2054A61K31/7072A61K47/38
Inventor 易崇勤王海超冀蕾
Owner PEKING UNIV FOUNDER GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com