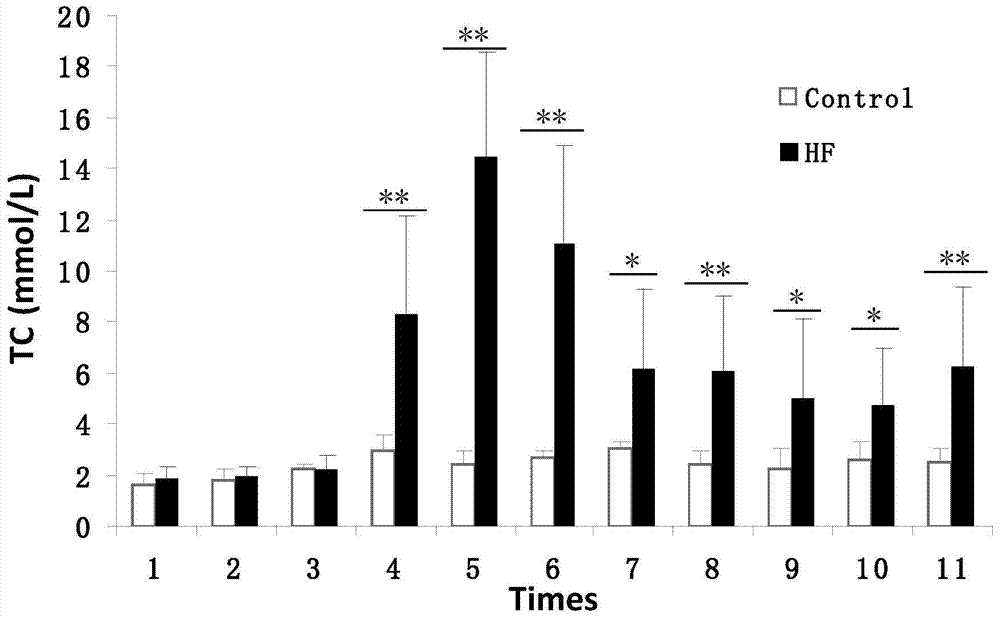
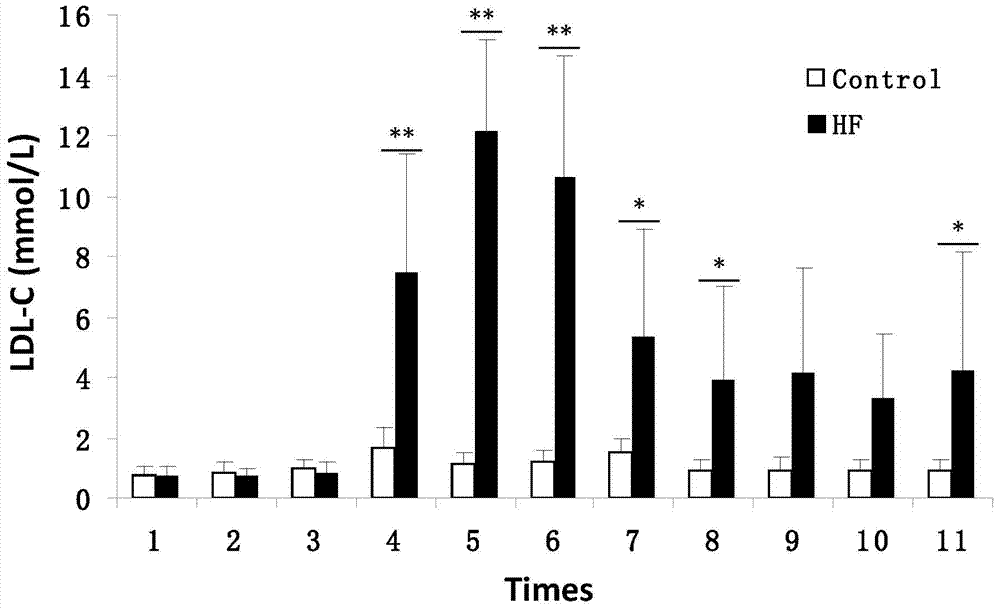
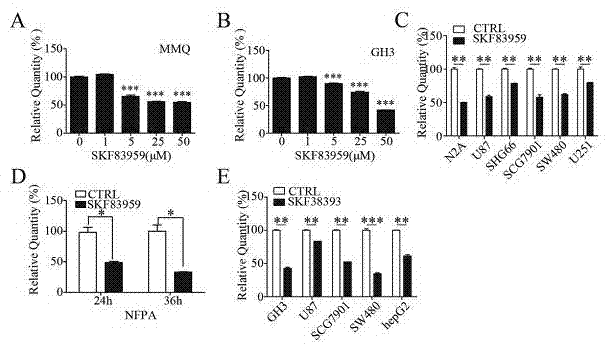
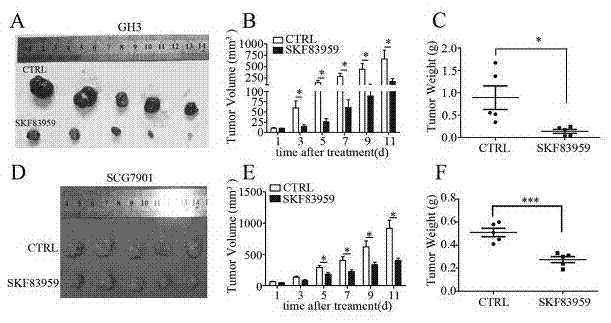
Patents
Literature
36 results about "Pharmacological interventions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmacological Intervention Definition. PHARMACOLOGICAL INTERVENTIONS. Drug therapy can be used to combat feelings of anxiety in an attempt to improvethe person’s quality of life. It is worth remembering that the use of prescribedmedication is not usually the first line of treatment, as other methods of cares will be considered initially.
Method of treating neurological diseases and etiologically related symptomology using carbonyl trapping agents in combination with medicaments
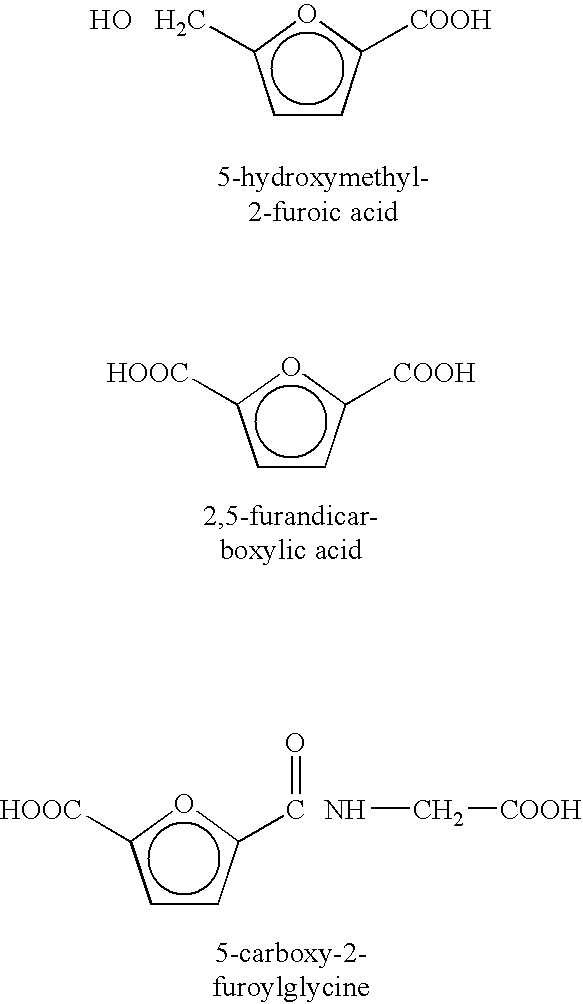
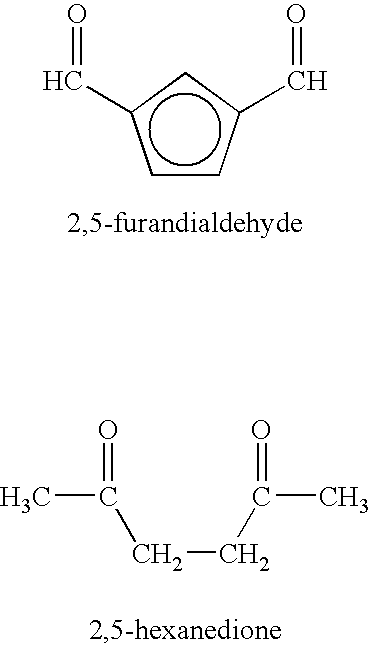
This invention defines a novel method for treatment of several neurological diseases and pathophysiologically related symptomology, said diseases including peripheral neuropathies, secondary symptomology of diabetes, Alzheimer's disease, Parkinson's disease, alcoholic polyneuropathy and age-onset symptomology, as well as analogous veterinary disease states. An opportunity exists for pharmacological intervention in some neurological diseases by use of water soluble, small molecular weight primary amine agents and chemical derivatives thereof. Examples of such primary pharmacological agents include 4-aminobenzoic acid and derivatives thereof. The present invention also includes: (1) oral use of optional non-absorbable polyamine polymeric co-agents such as chitosan, (2) oral use of optional known antioxidant co-agents and nutritional factors related thereto, and (3) use of the primary agents and co-agents noted above in optional combination with medicaments recognized as effective for treatment of the diseases addressed herein or symptoms thereof.
Owner:SECANT PHARMA
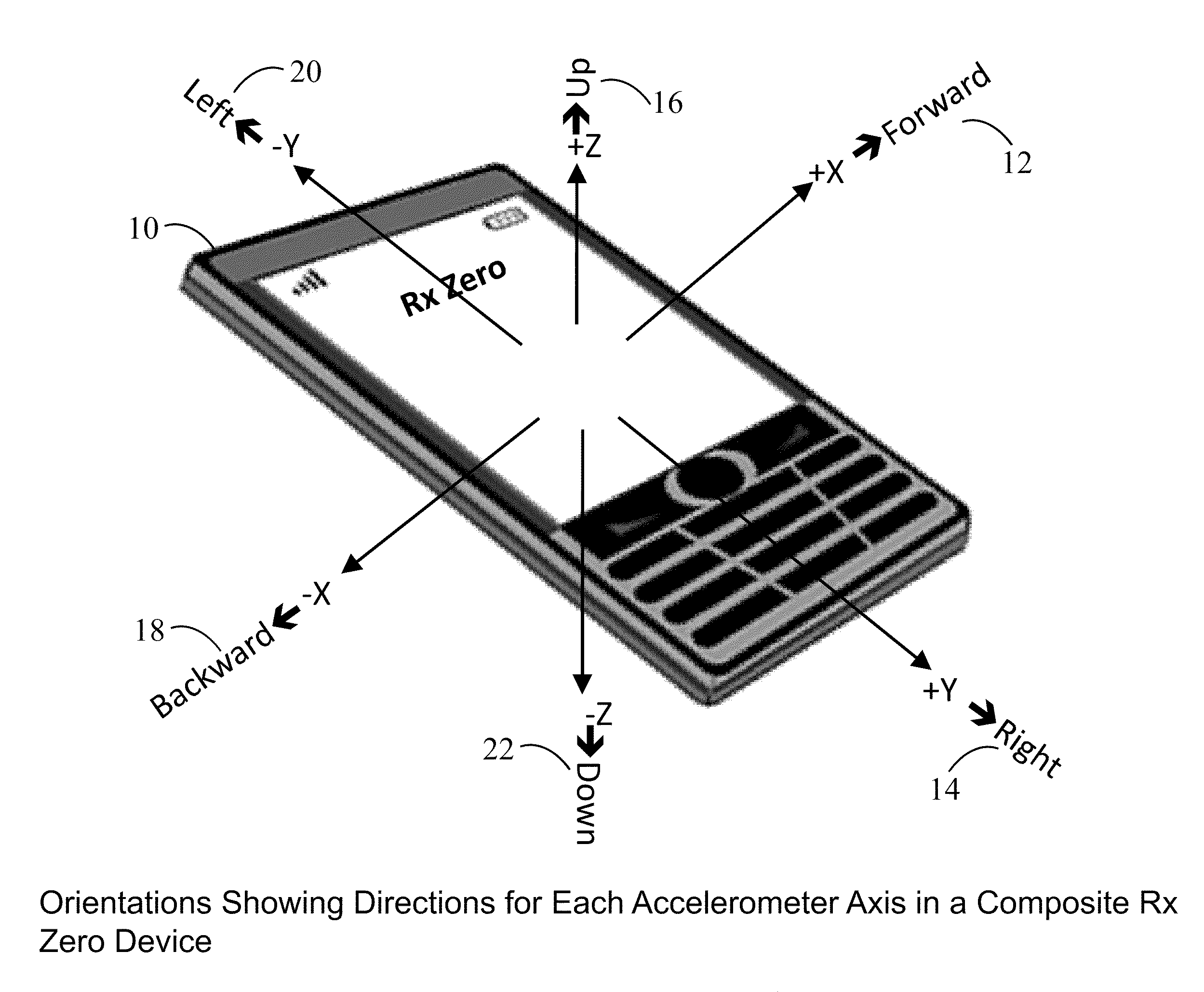
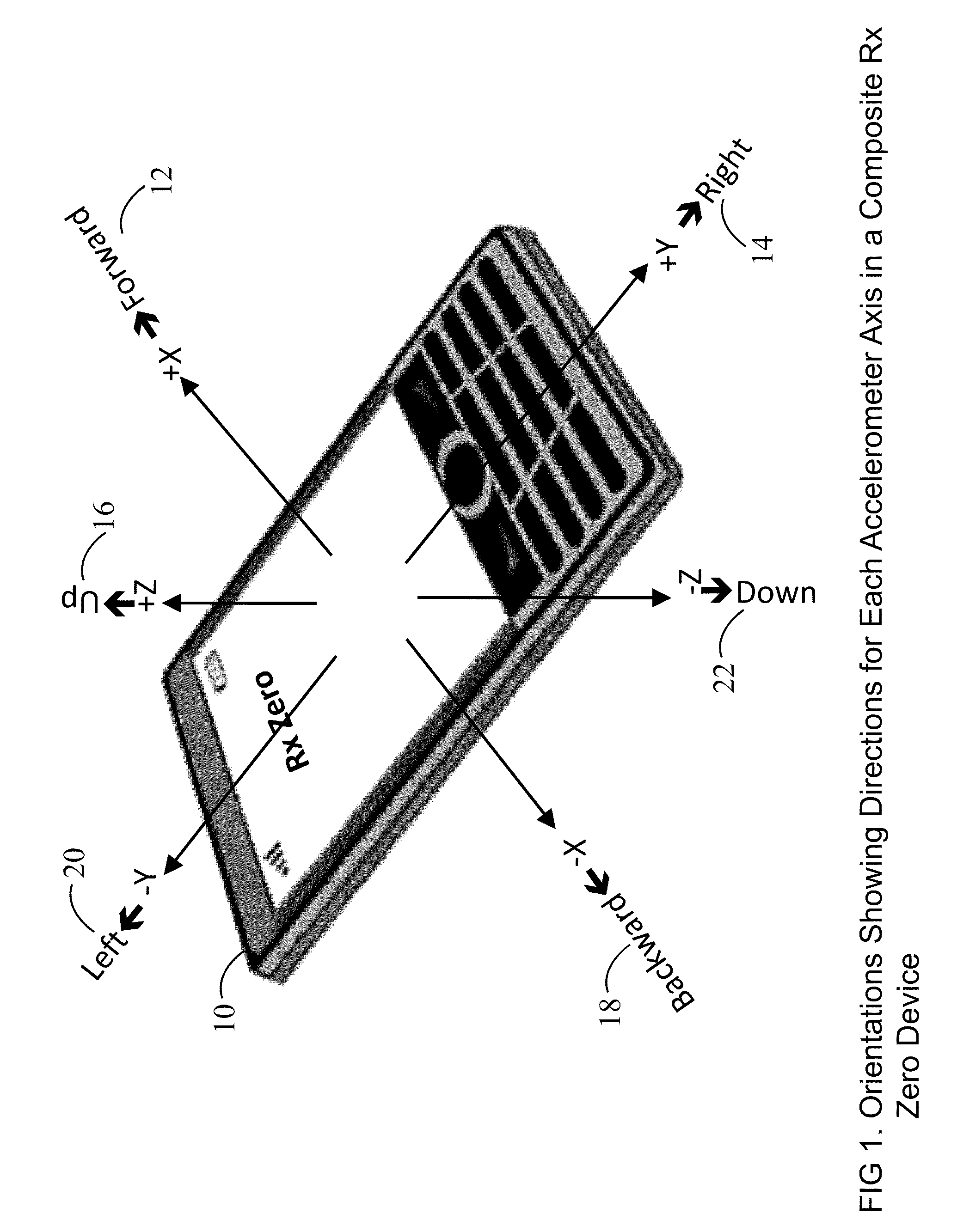
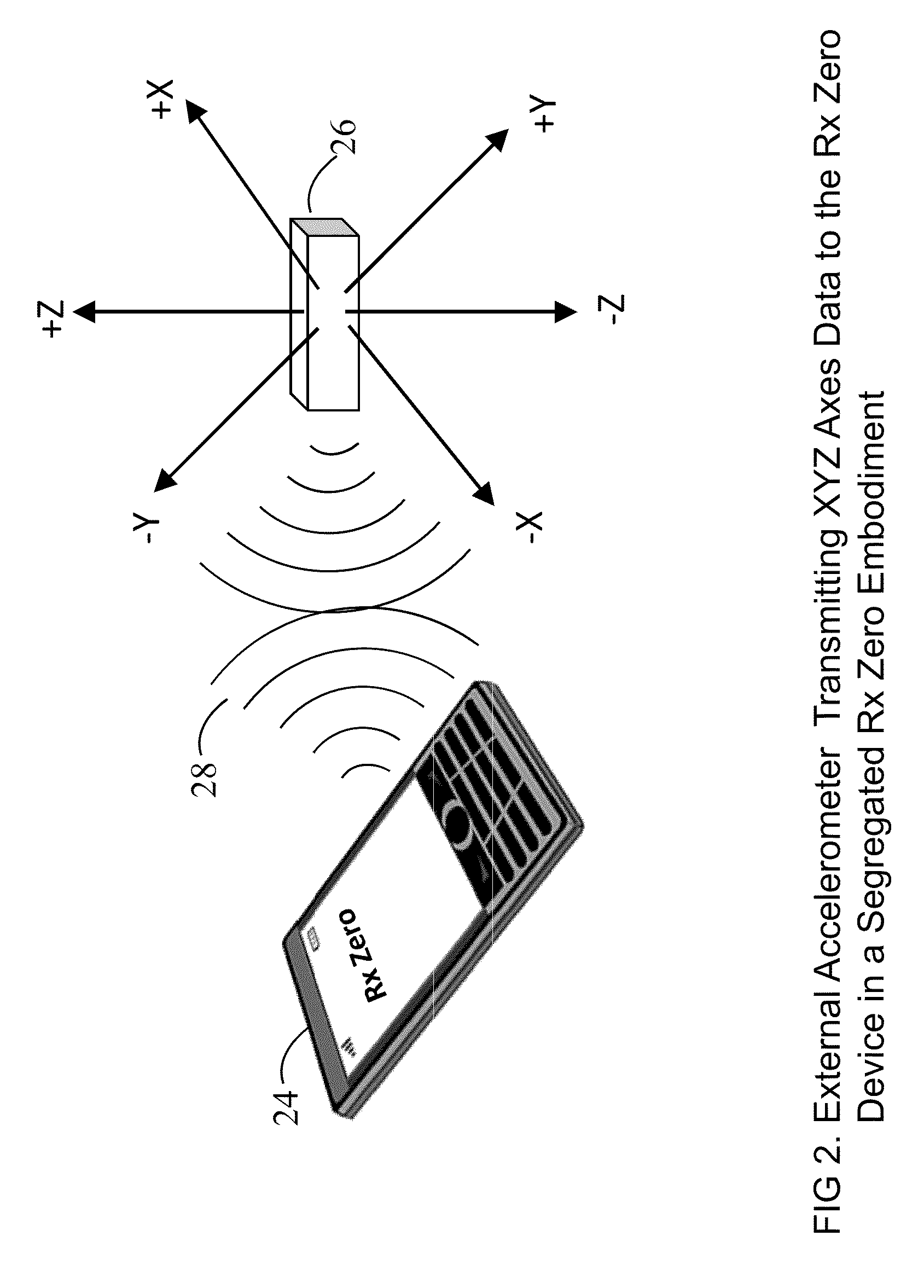
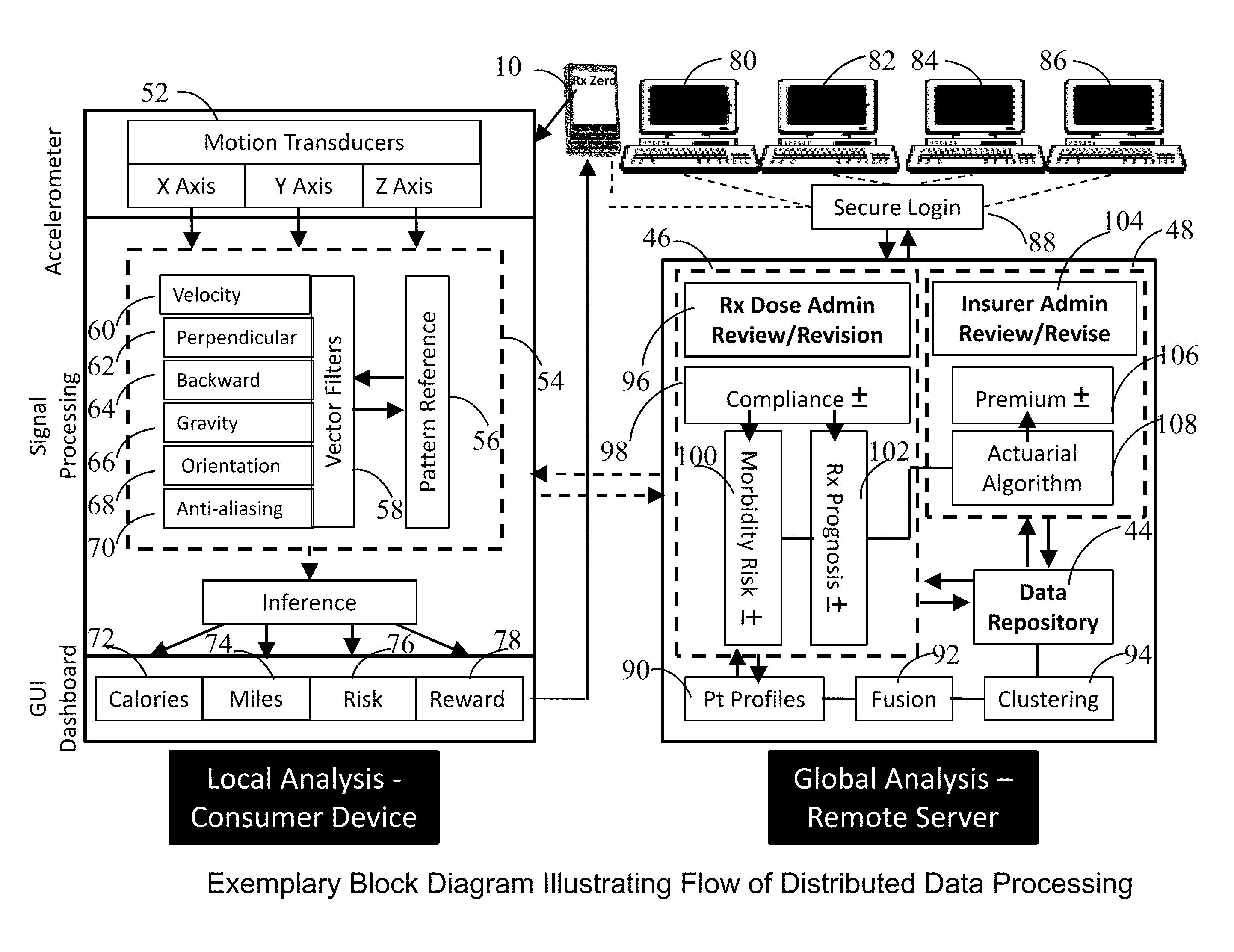
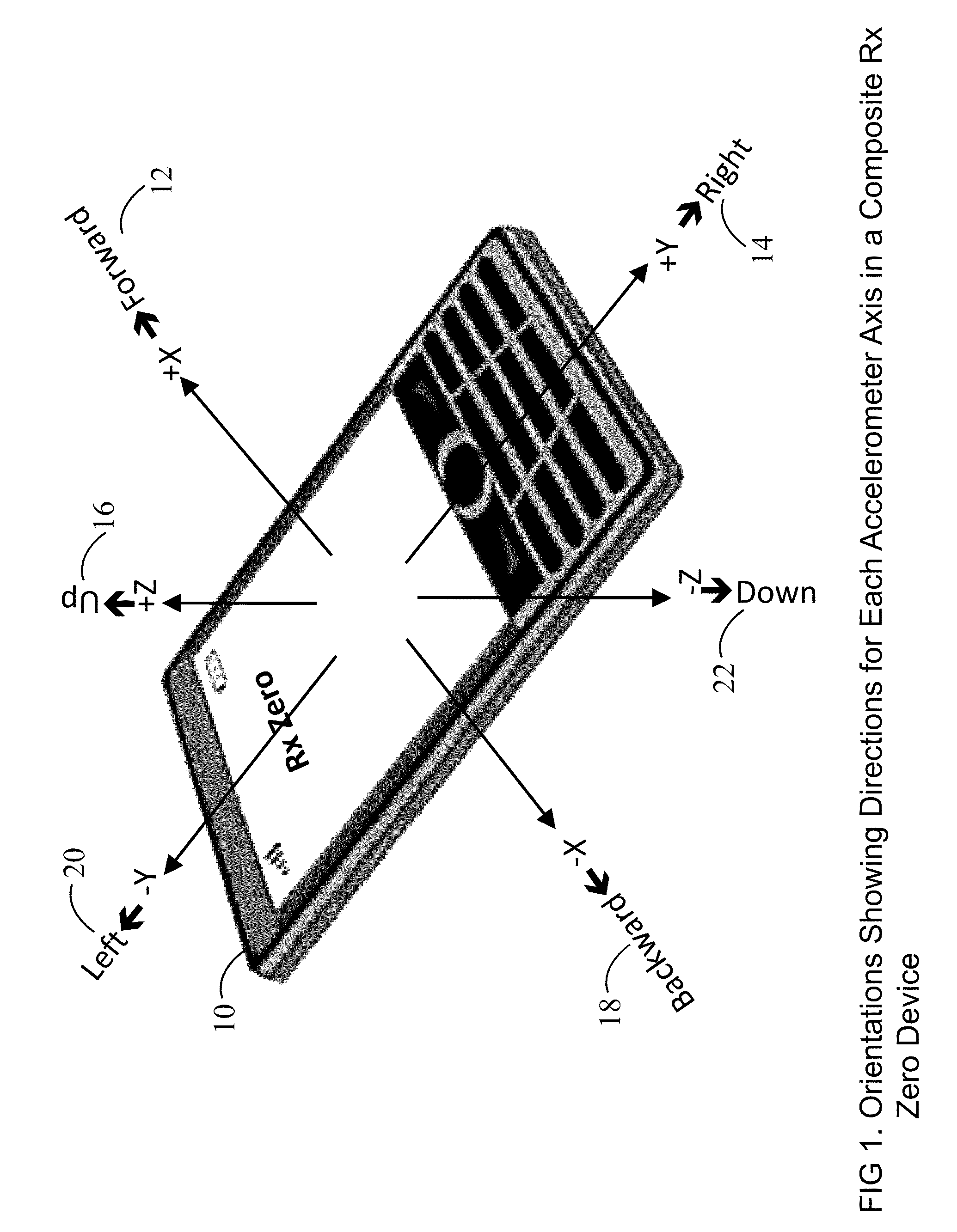
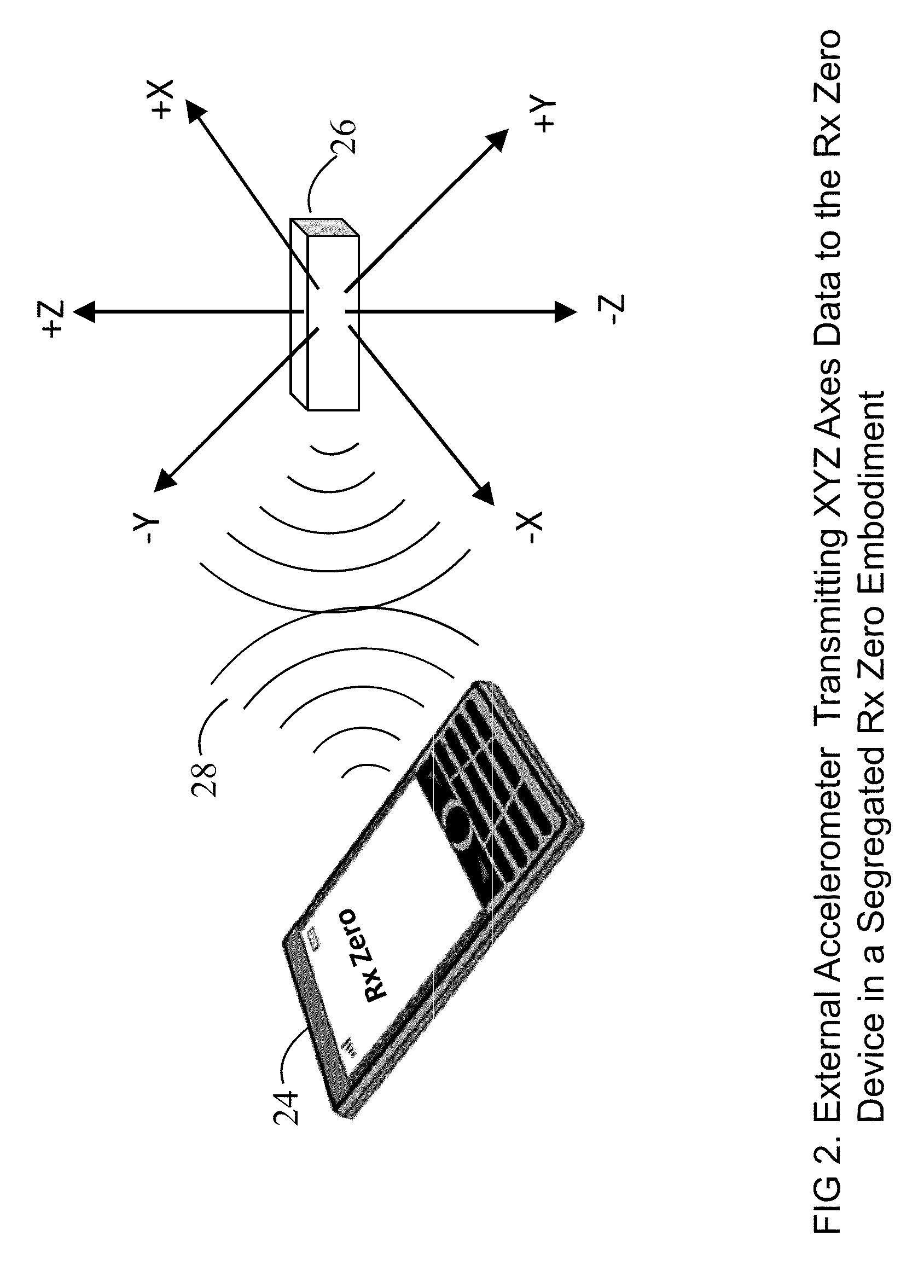
Prescription Zero: A non-pharmaceutical prescription device for prescribing, administering, monitoring, measuring and motivating a therapeutic lifestyle regimen for prevention and treatment of chronic diseases
InactiveUS20110046519A1Good for healthAlter and maintain healthy lifestylePerson identificationInertial sensorsRegimenPharmacological interventions
The invention discloses a wearable / handheld personal communication device with hardware and software sensor modules that sense and analyze all caregiver prescribed / monitored user-lifestyle activities, and deploys such analysis in improving user's overall health in terms of reduced risks for all-cause morbidities / mortalities and eventually a life without drugs. Termed Rx Zero, such method may be prescribed not just for maintaining a healthy lifestyle, but for treatment of chronic diseases with intent to wean the patients to minimal or zero pharmacological intervention, or in combination with medications to improve prognosis of the disease under treatment.The benefits of the Rx Zero method of the present invention extend not only to the individual and the community through high quality healthcare at lower cost, but payers by reducing the loss ratio on account of reduced cost of medical claims, and to the caregivers in terms of an effective tool that disseminates, implements and redefines “Primary Health Care” and “Prevention” at levels beyond the terms' currently understood scope that has transformed healthcare to sickcare.
Owner:RAHEMAN FAZAL
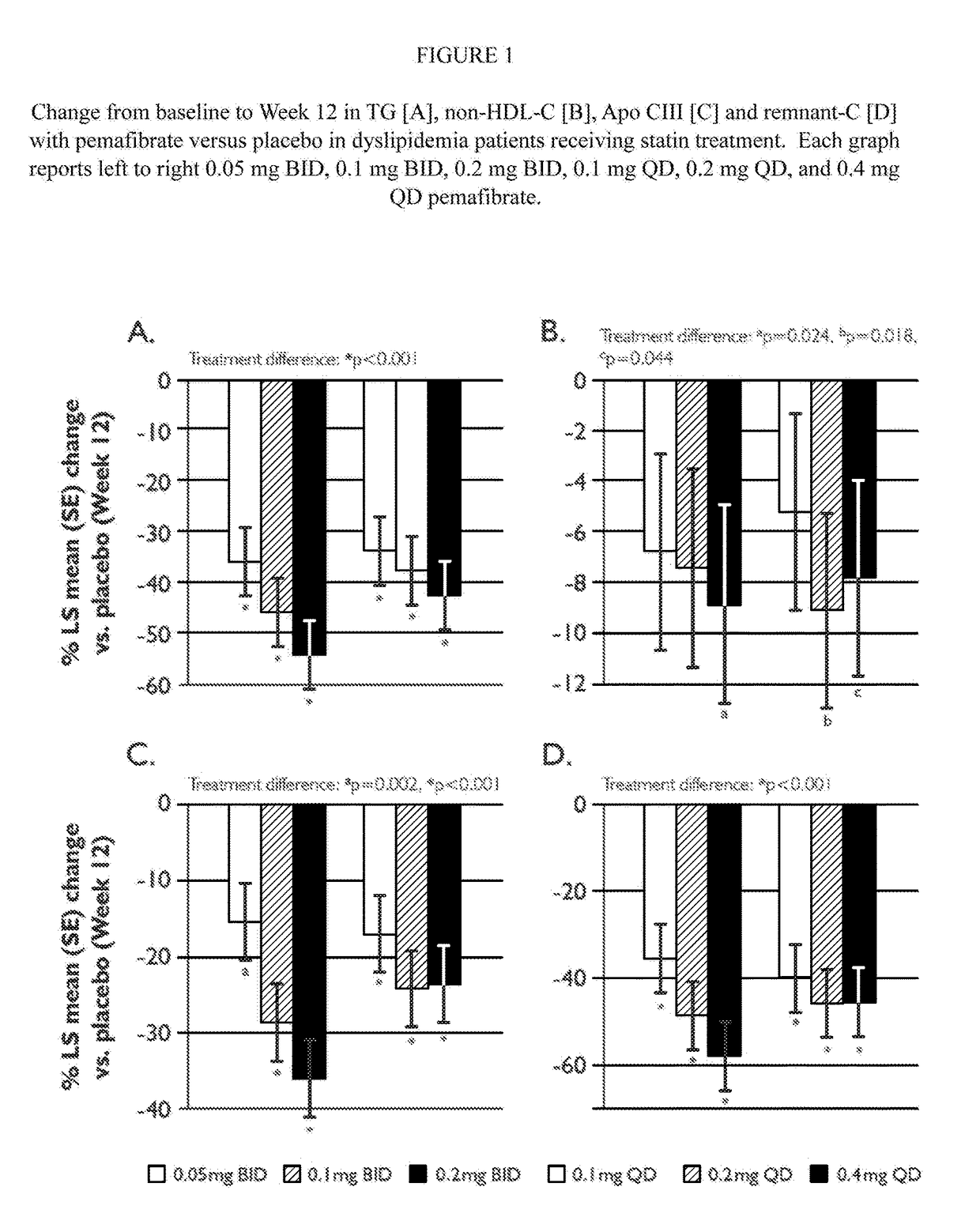
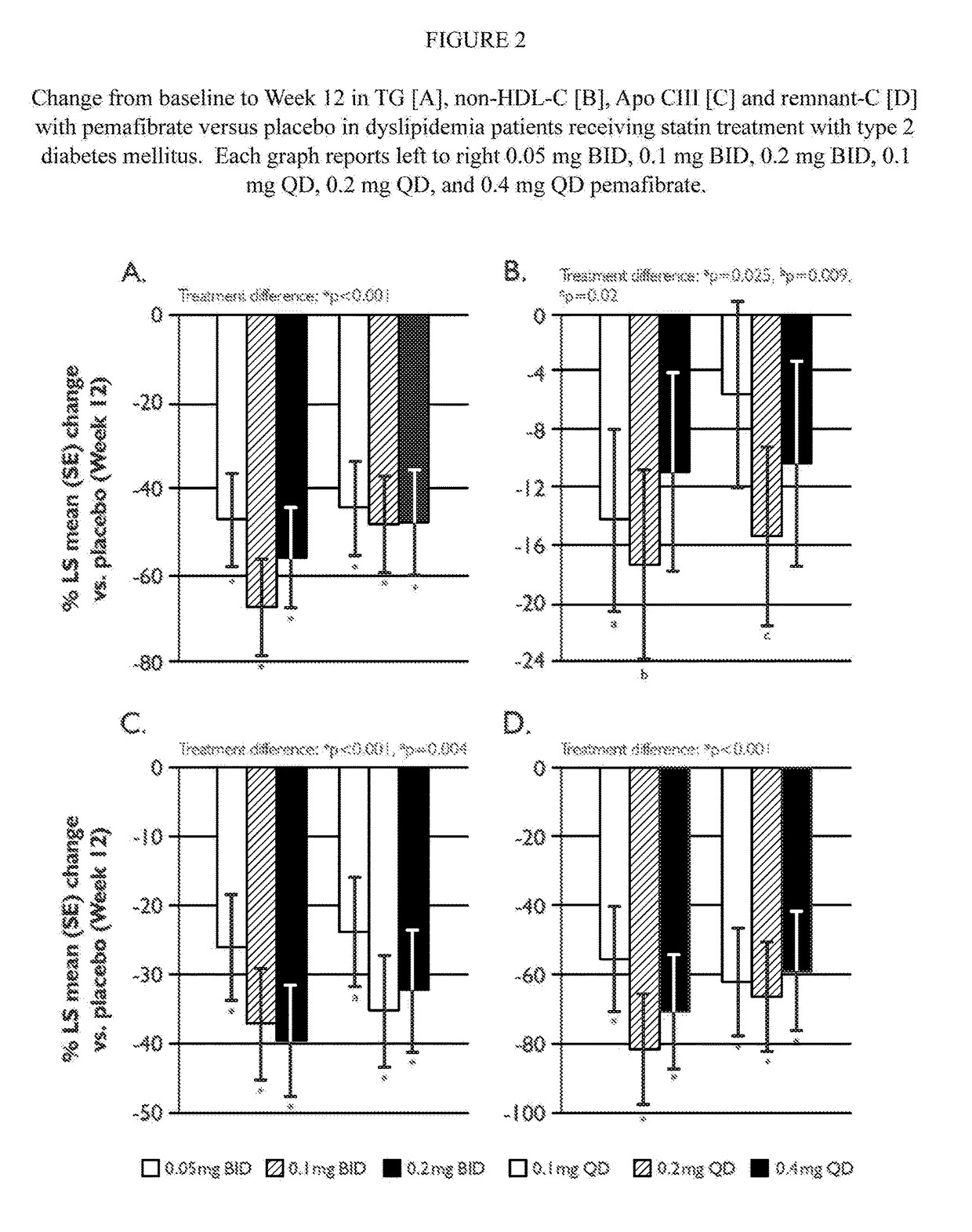
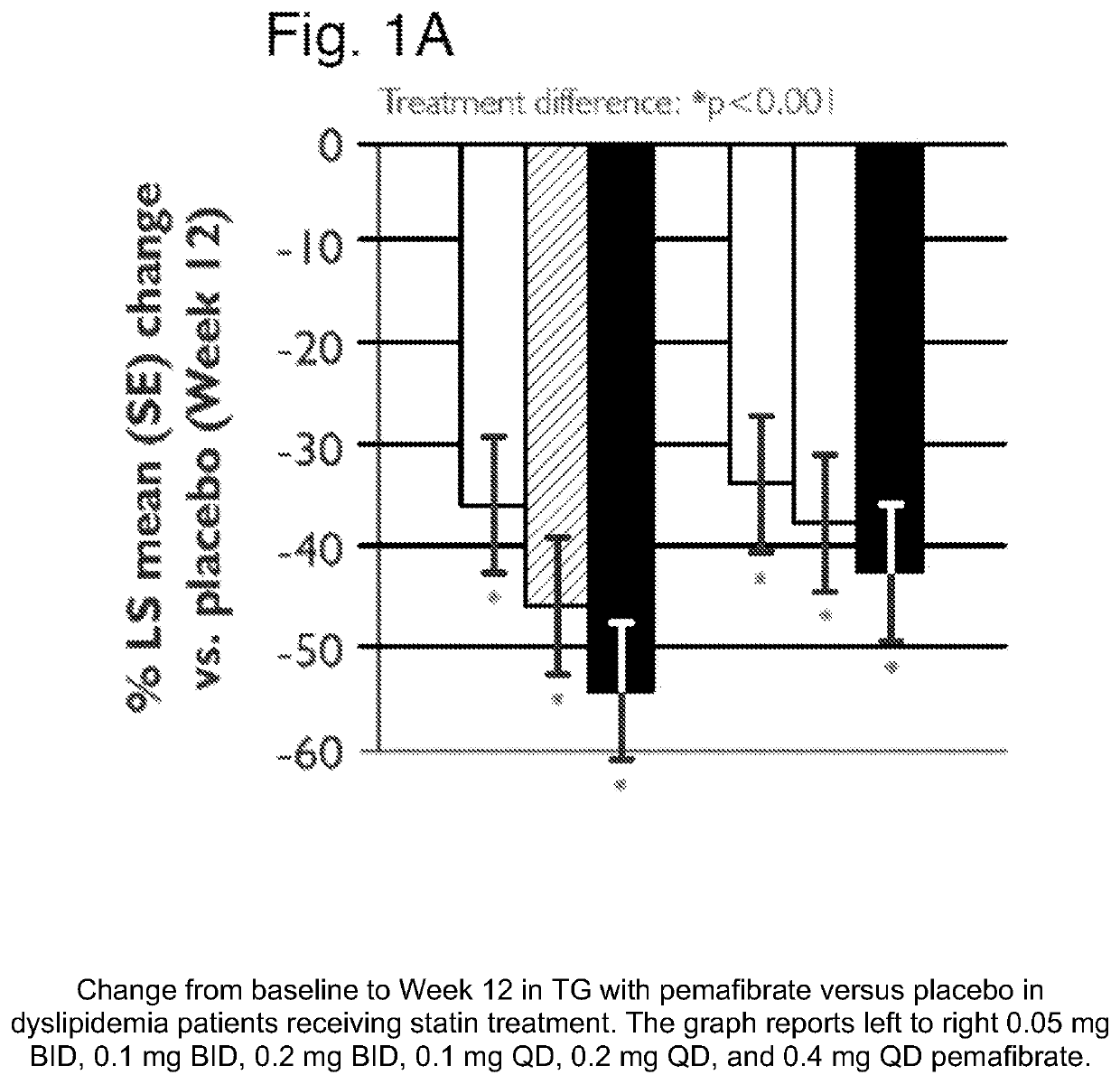
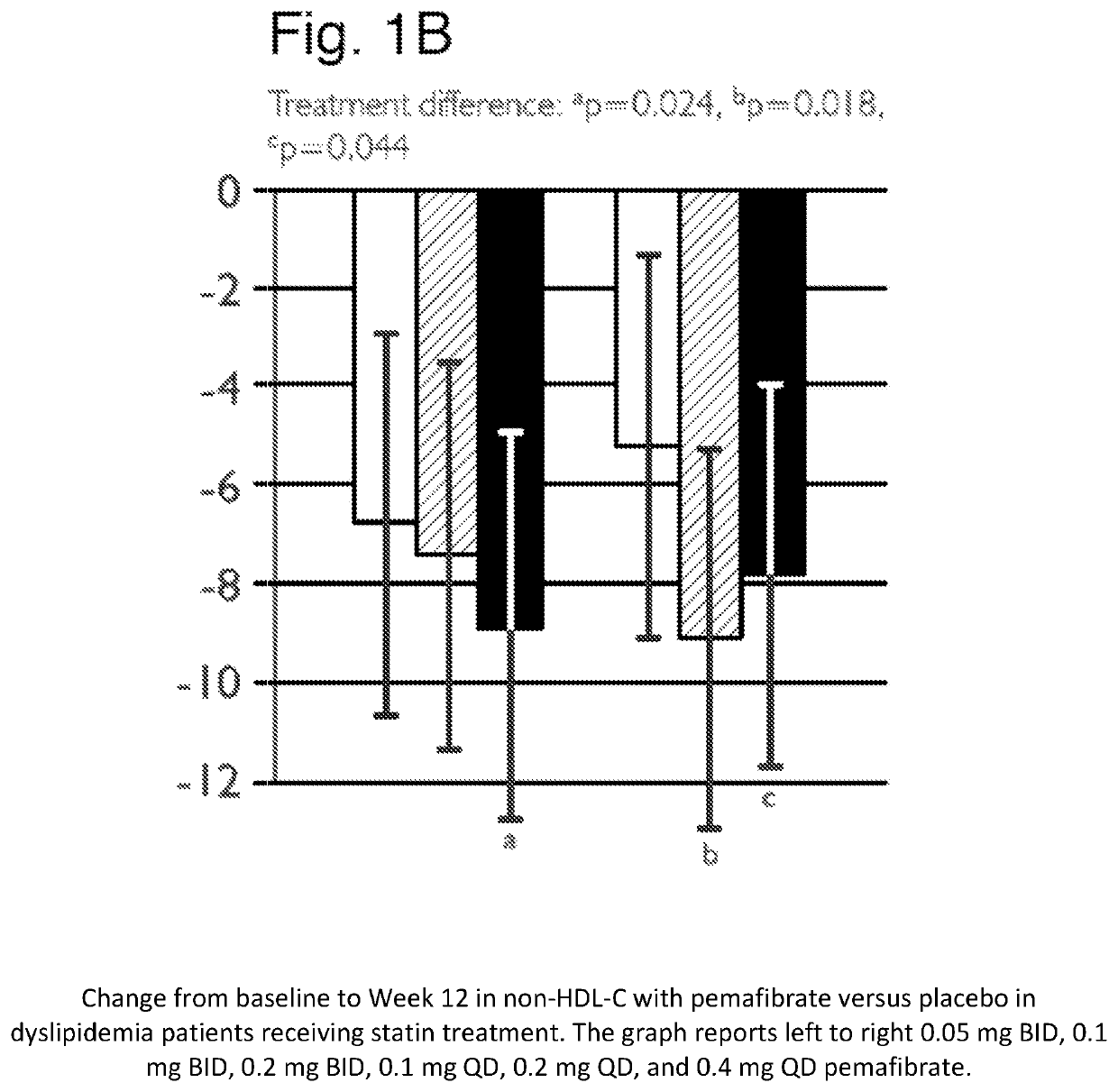
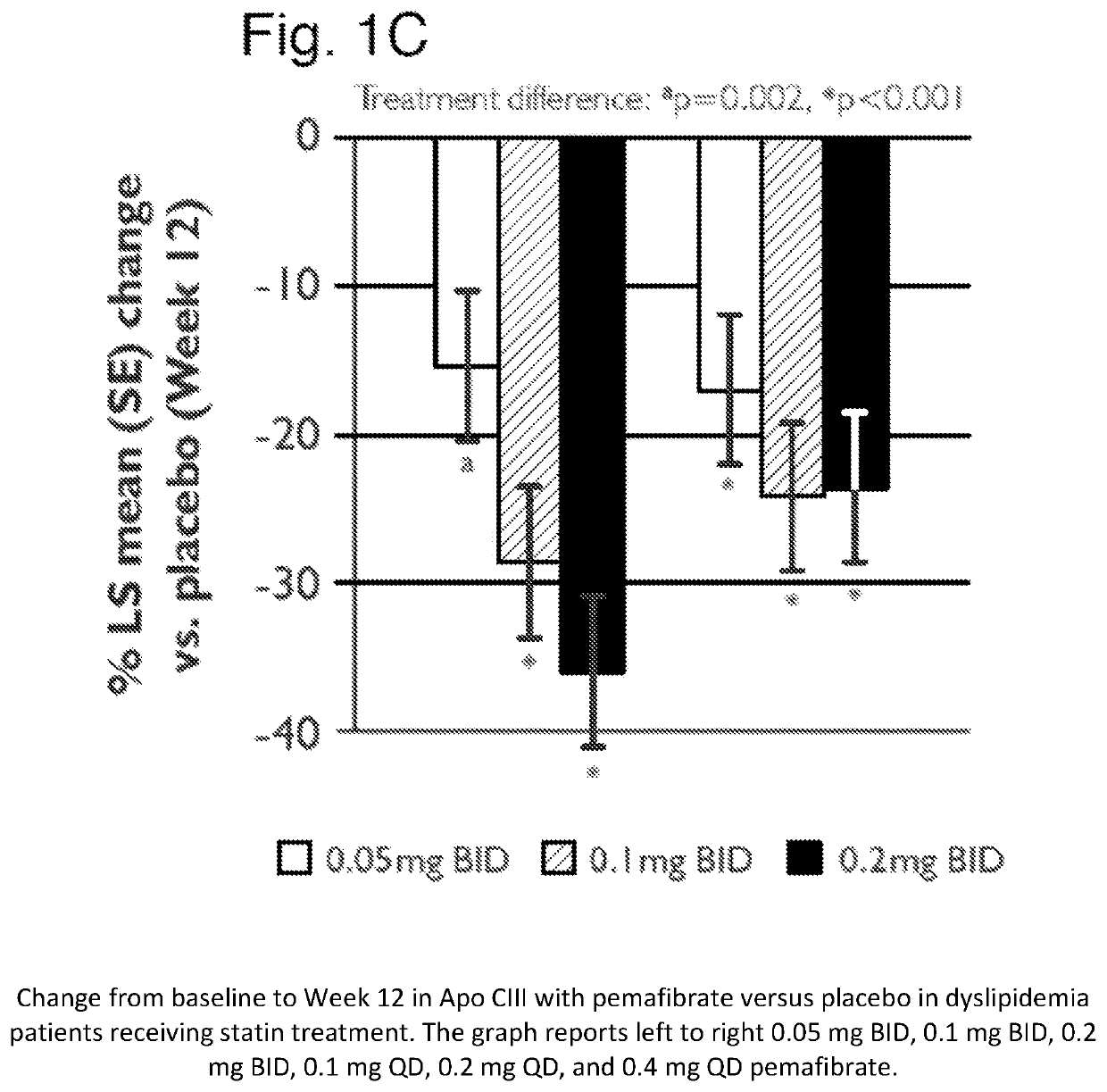
Methods of Preventing Cardiovascular Events in Residual Risk Dyslipidemic Populations
ActiveUS20180028505A1Reduce cardiovascular riskReduce riskMetabolism disorderPharmaceutical delivery mechanismDyslipidemiaPharmacological interventions
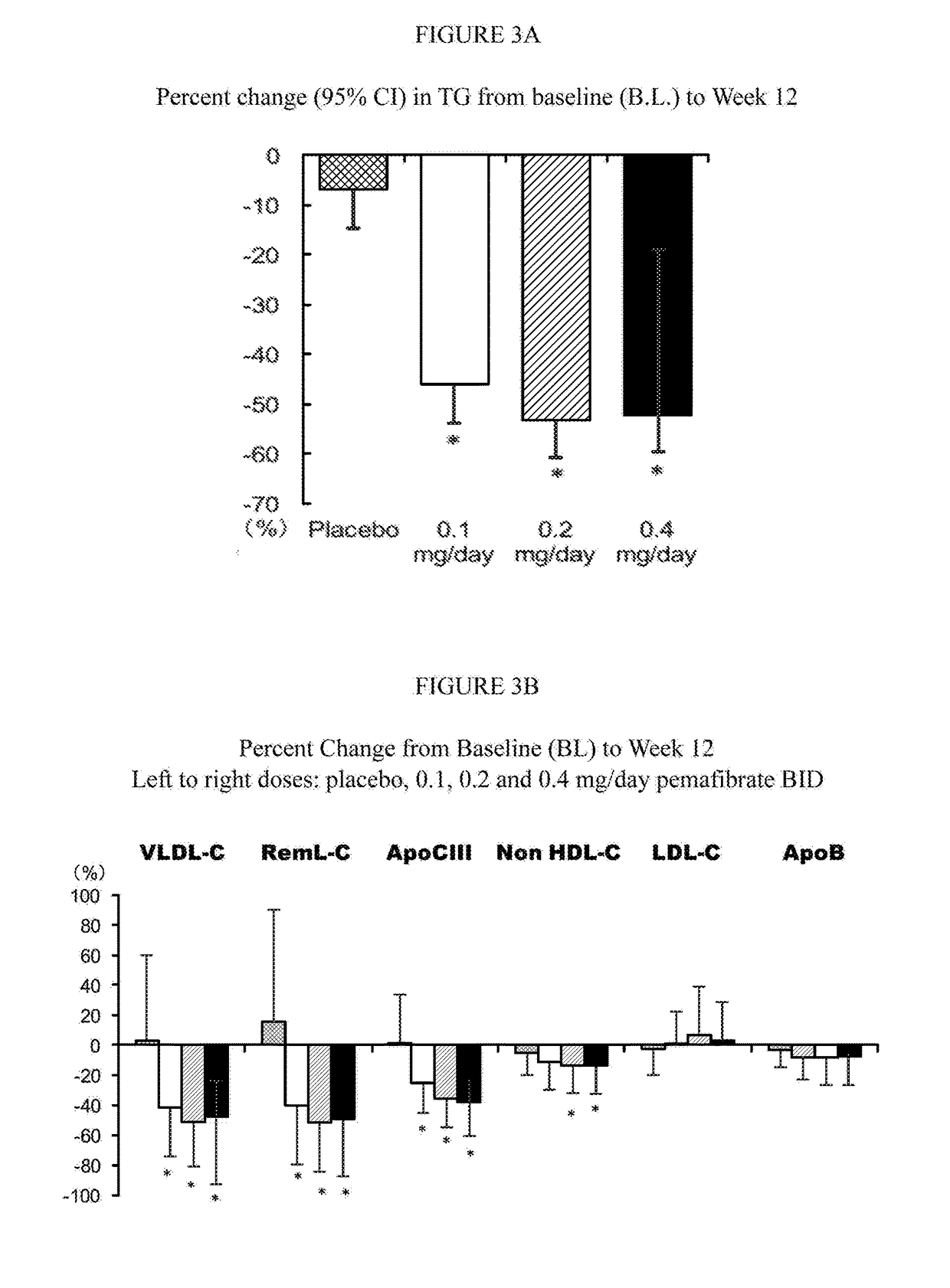
The present invention provides pharmacological interventions for the treatment of dyslipidemia, and to the reduction of residual risk of cardiovascular disease and adverse cardiovascular events in patients on intense statin use or with well-controlled LDL-C concentrations. In particular, the invention relates to the use of pemafibrate to prevent cardiovascular events in populations at-risk due to risk factors such as type 2 diabetes mellitus with dyslipidemia in spite of intense statin use or well-controlled LDL-C.
Owner:KOWA CO LTD
Methods for regulating inflammatory mediators and peptides useful therein
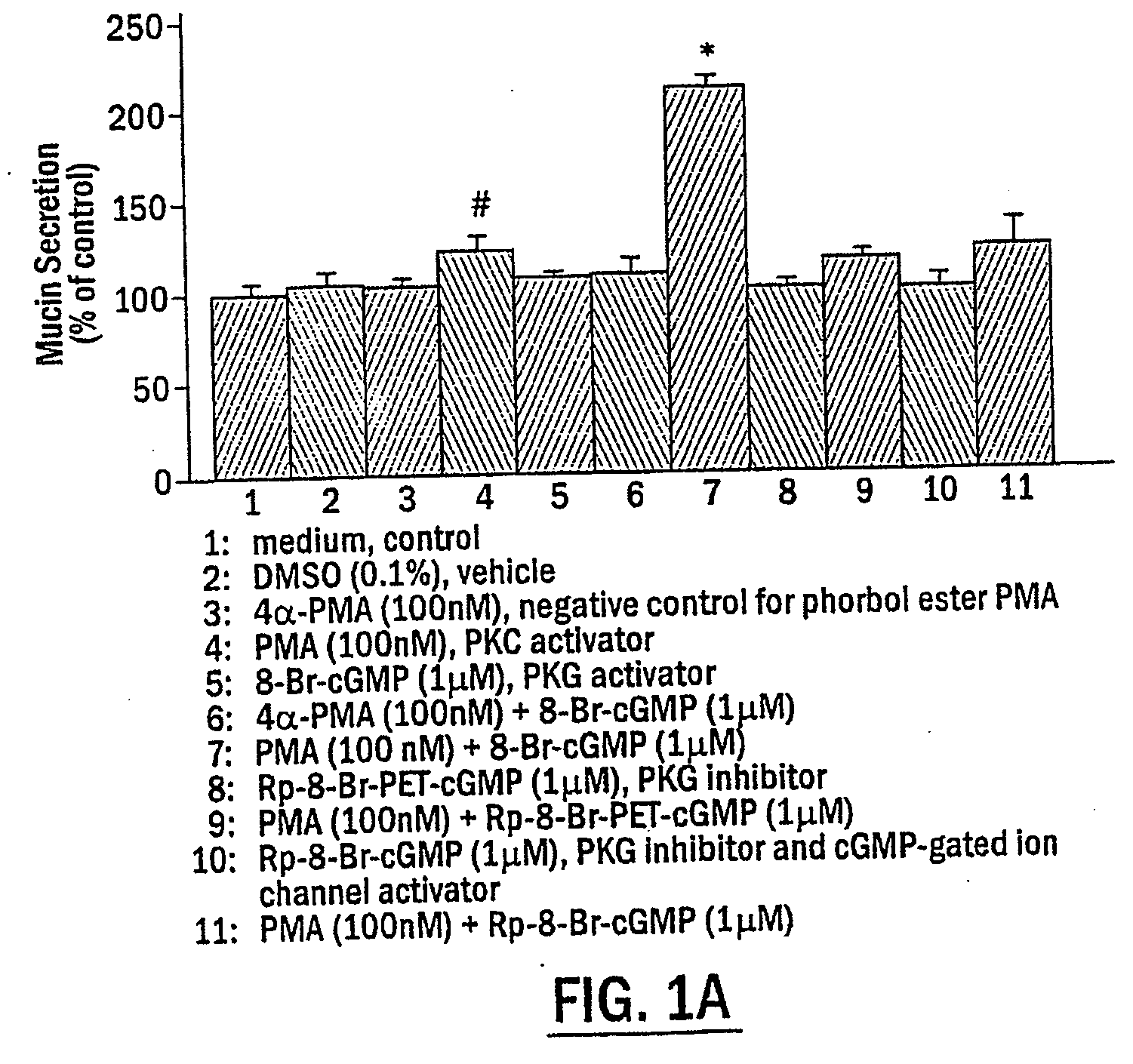
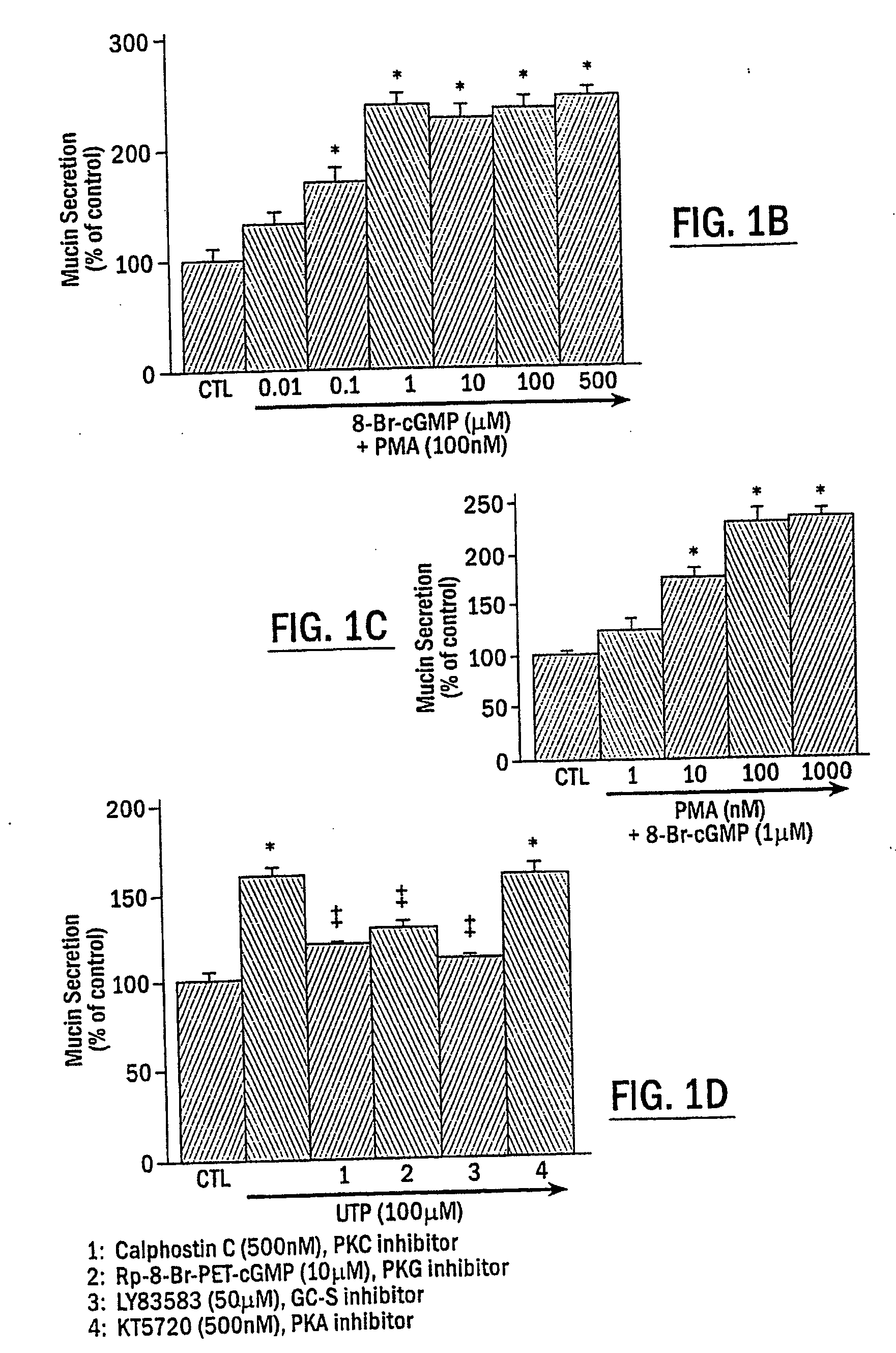
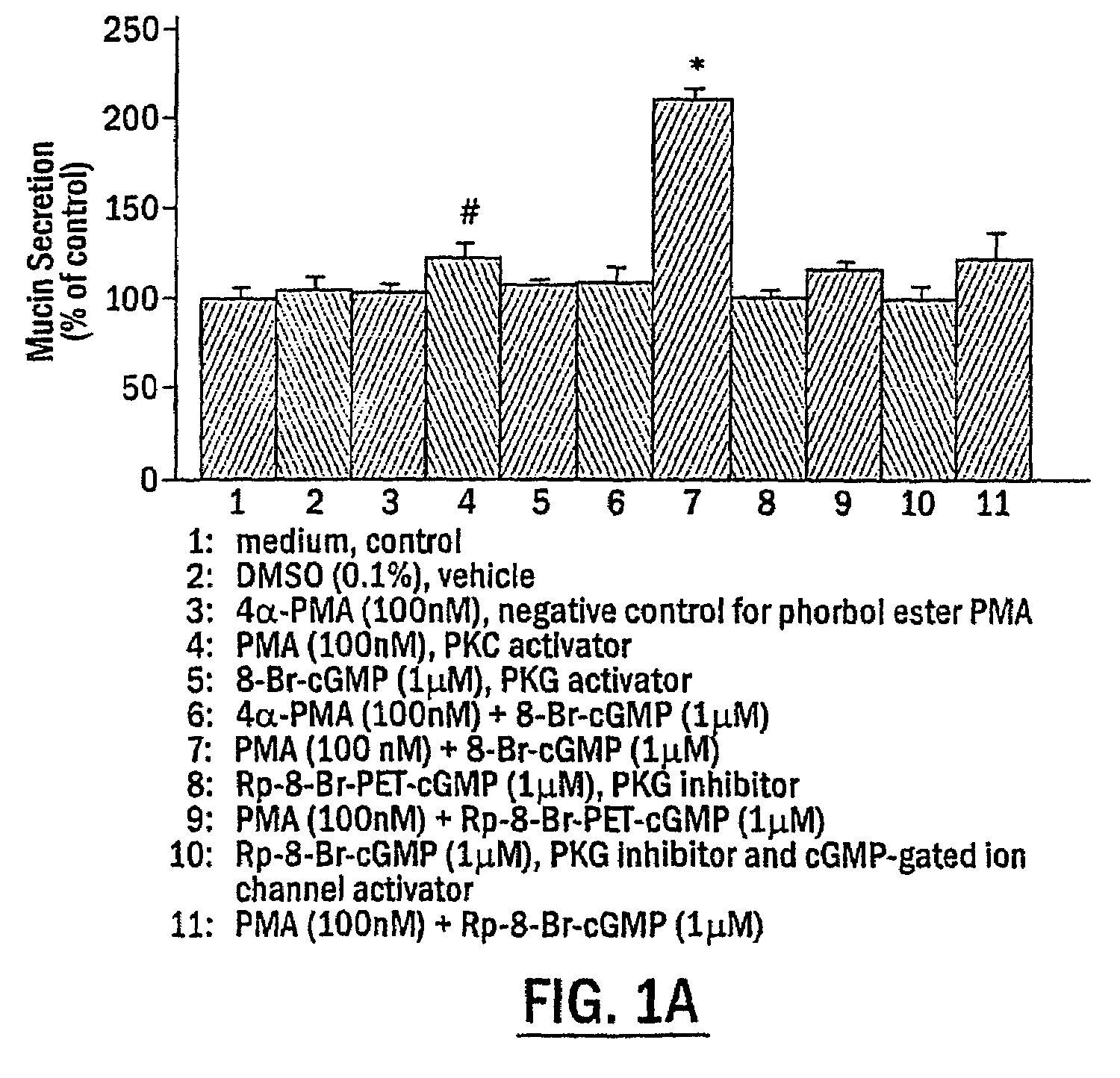
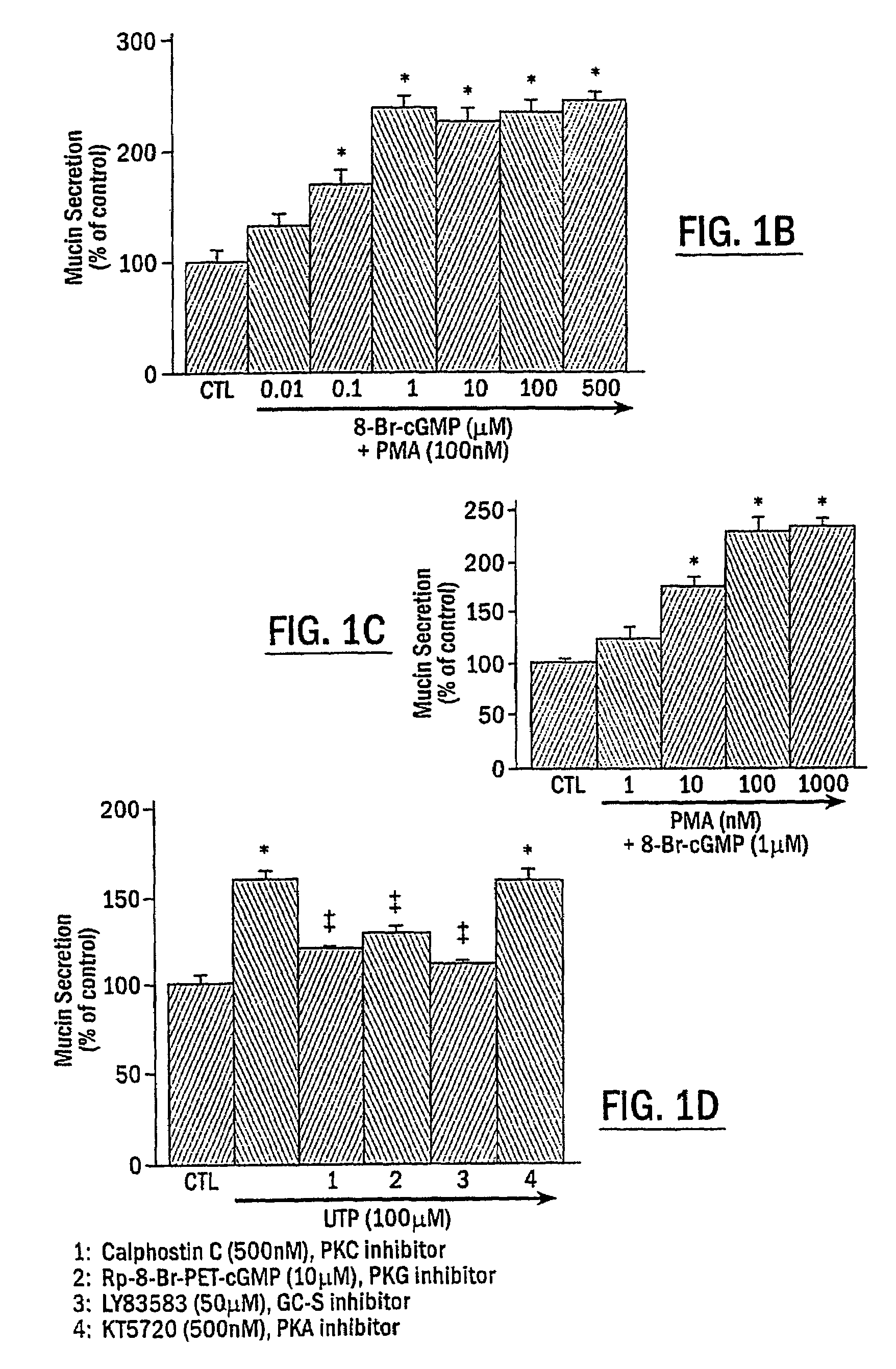
The present invention includes methods of modulating cellular secretory processes. More specifically the present invention relates to modulating or reducing the release of inflammatory mediators from inflammatory cells by inhibiting the mechanism associated with the release of inflammatory mediators from the vesicles or granules in the inflammatory cells. In this regard, the present invention discloses an intracellular signaling mechanism that illustrates several novel intracellular targets for pharmacological intervention in disorders involving secretion of inflammatory mediators from vesicles in inflammatory cells. MANS peptide and active fragments thereof are useful in such methods.
Owner:BIOMARK PHARMACEUTICALS LTD +1
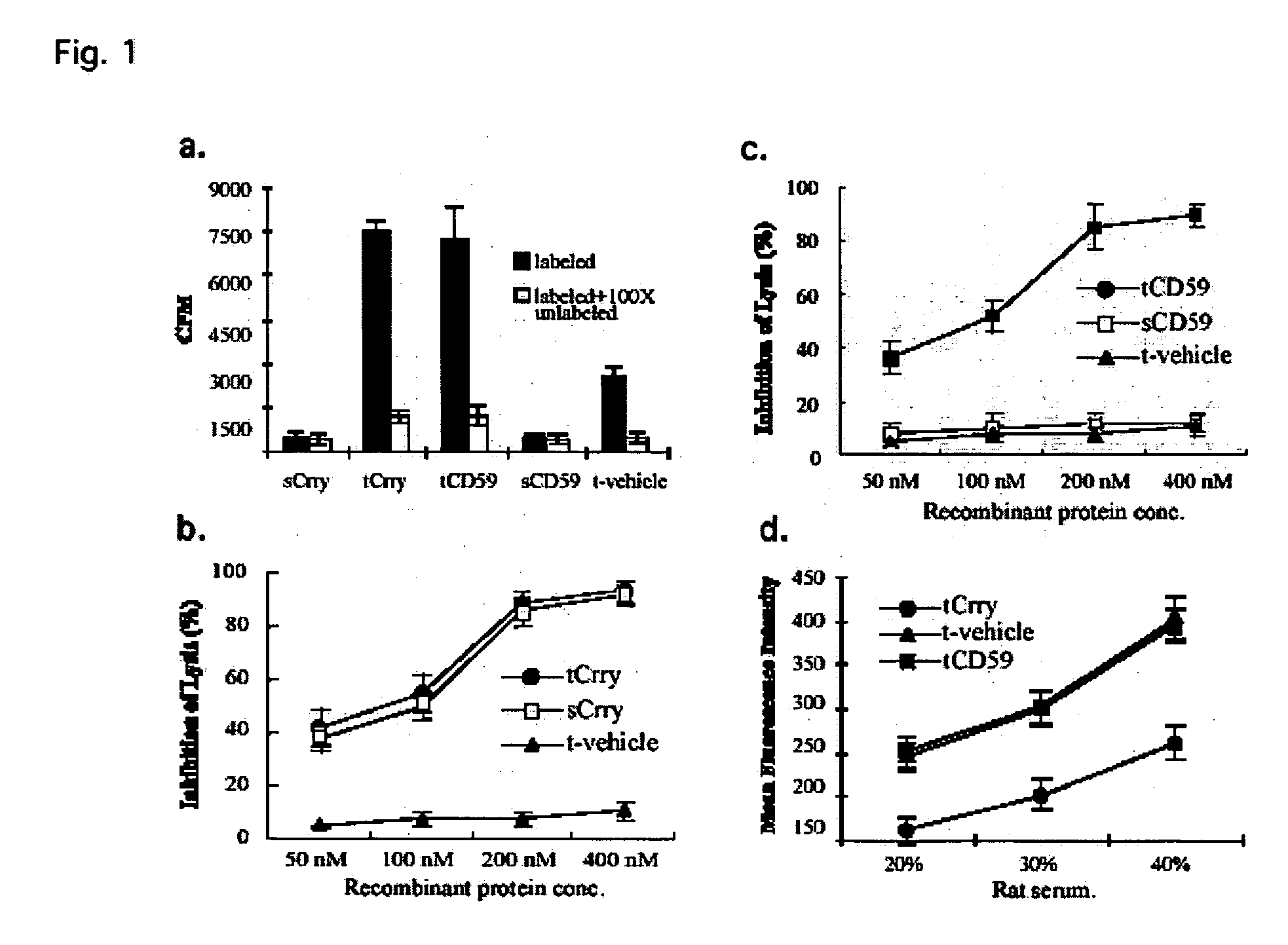
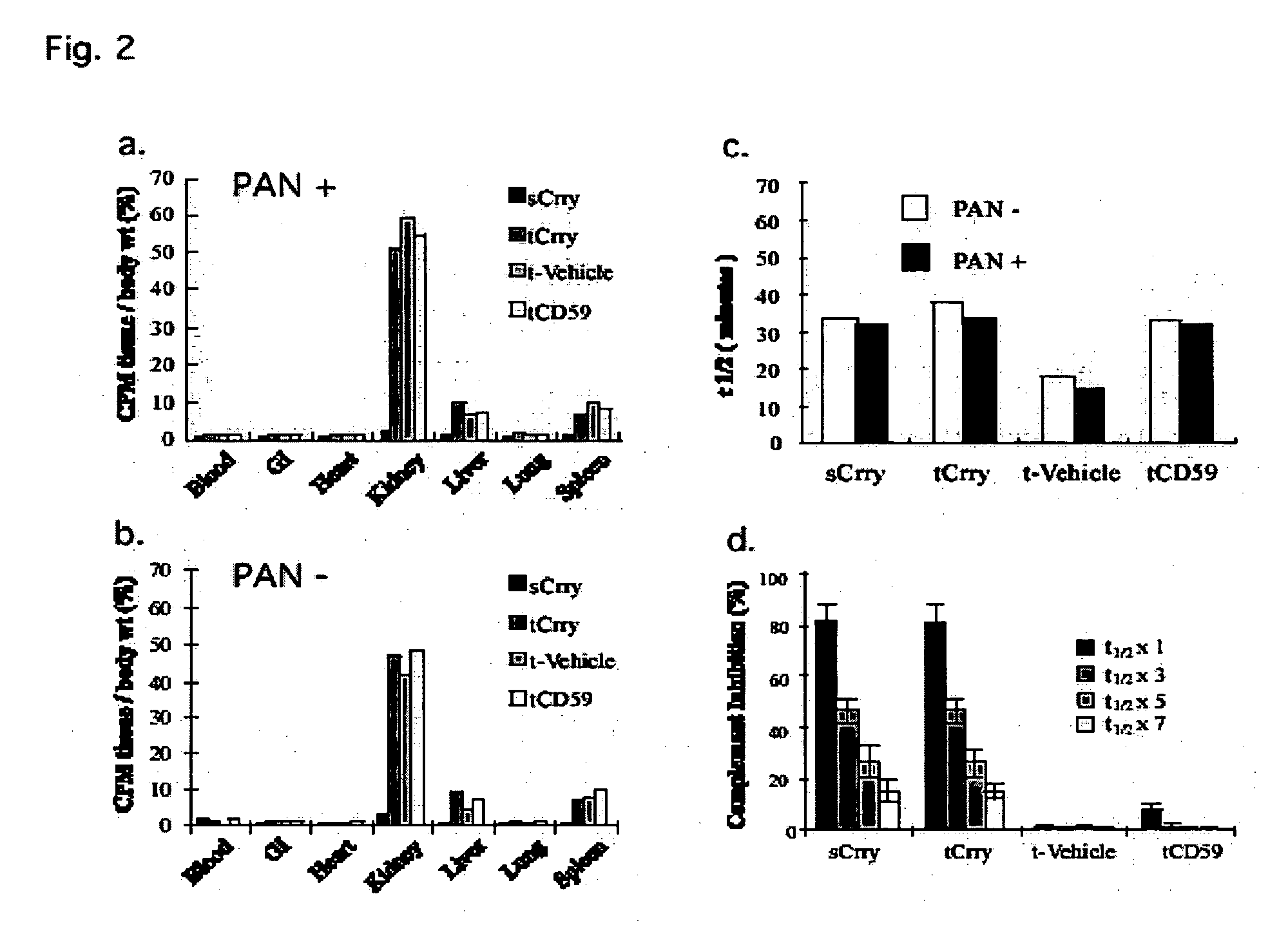
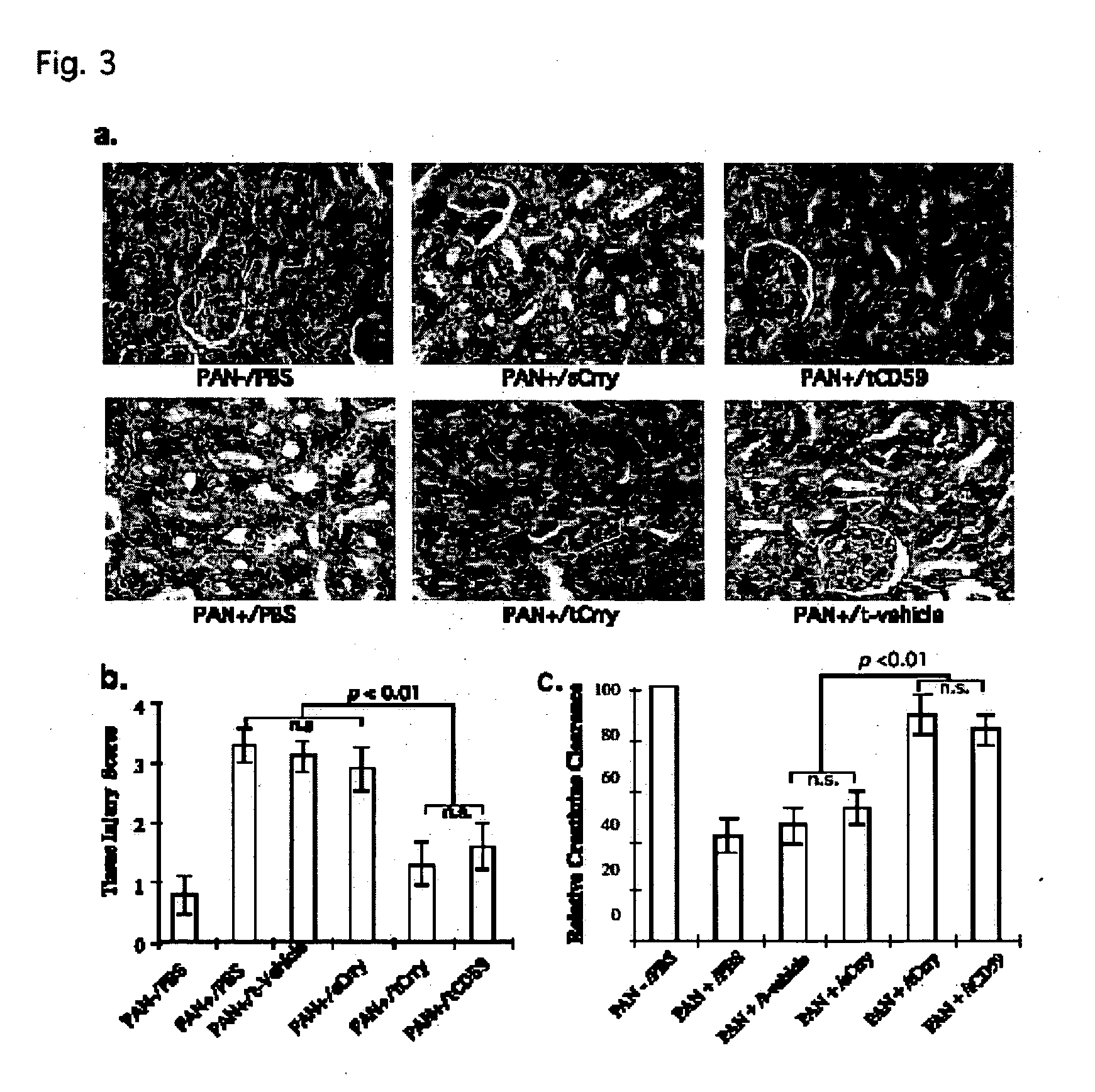
Tissue targeted complement modulators
InactiveUS20050265995A1Cell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsEpitheliumWhole body
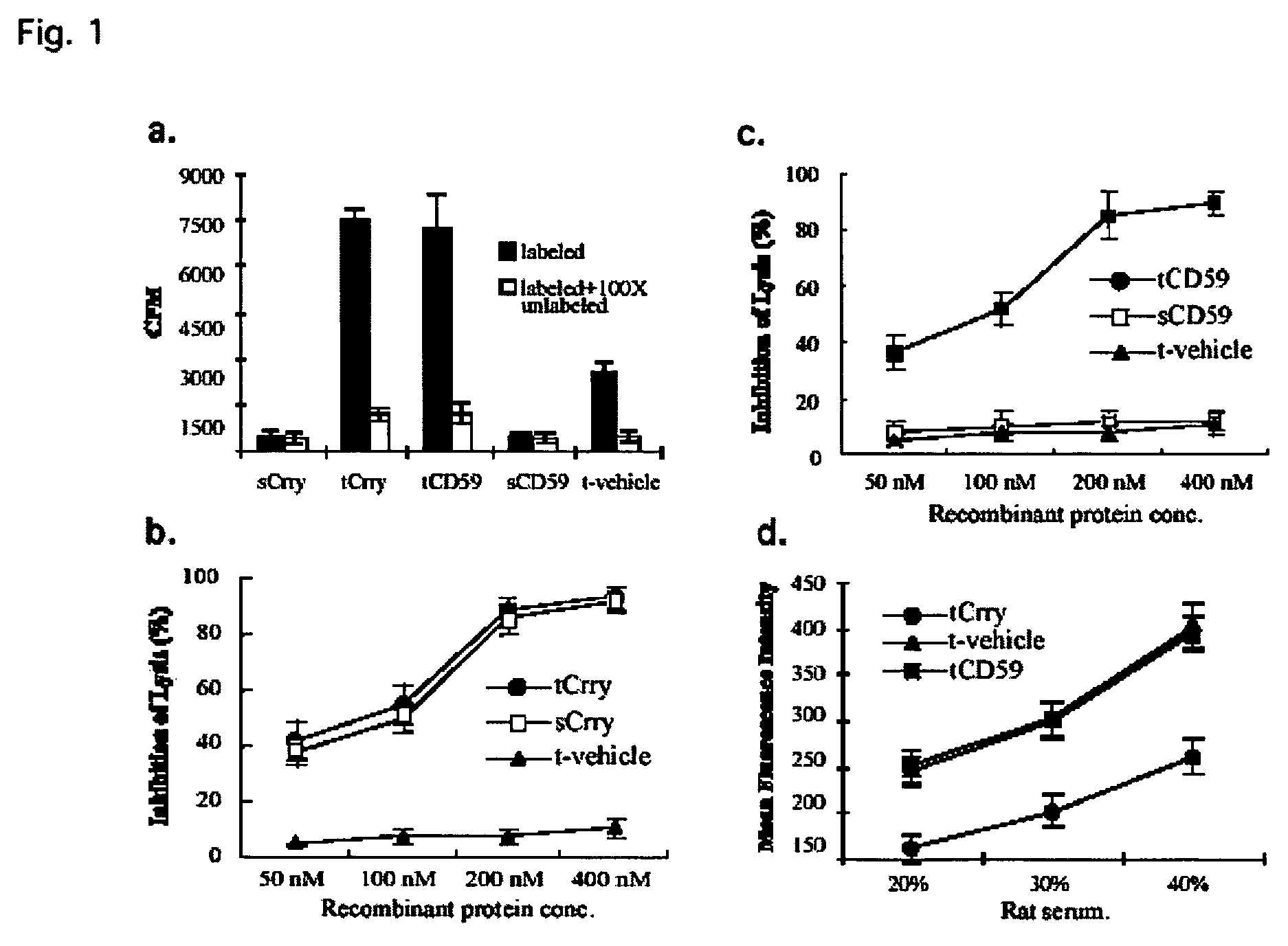
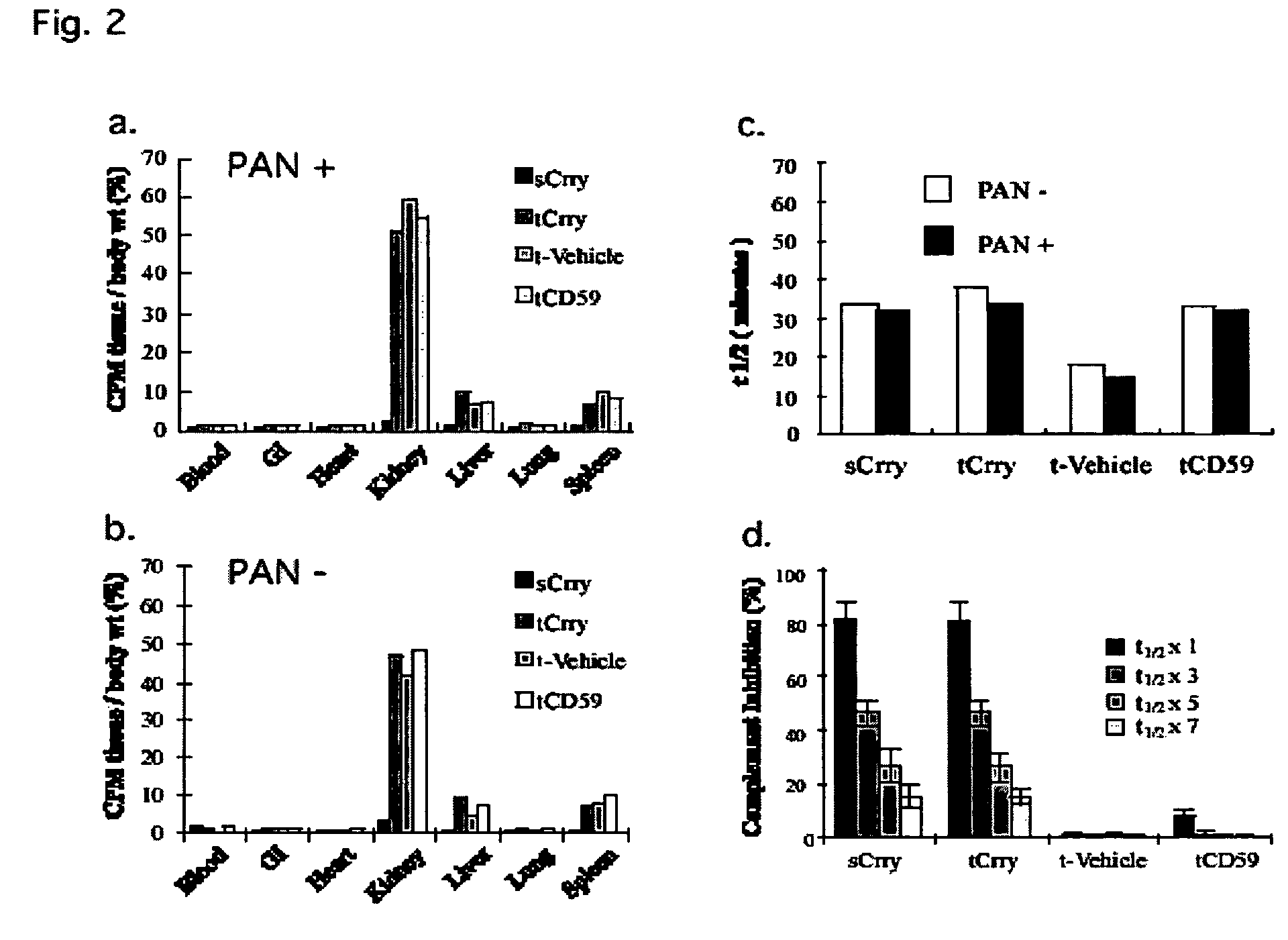
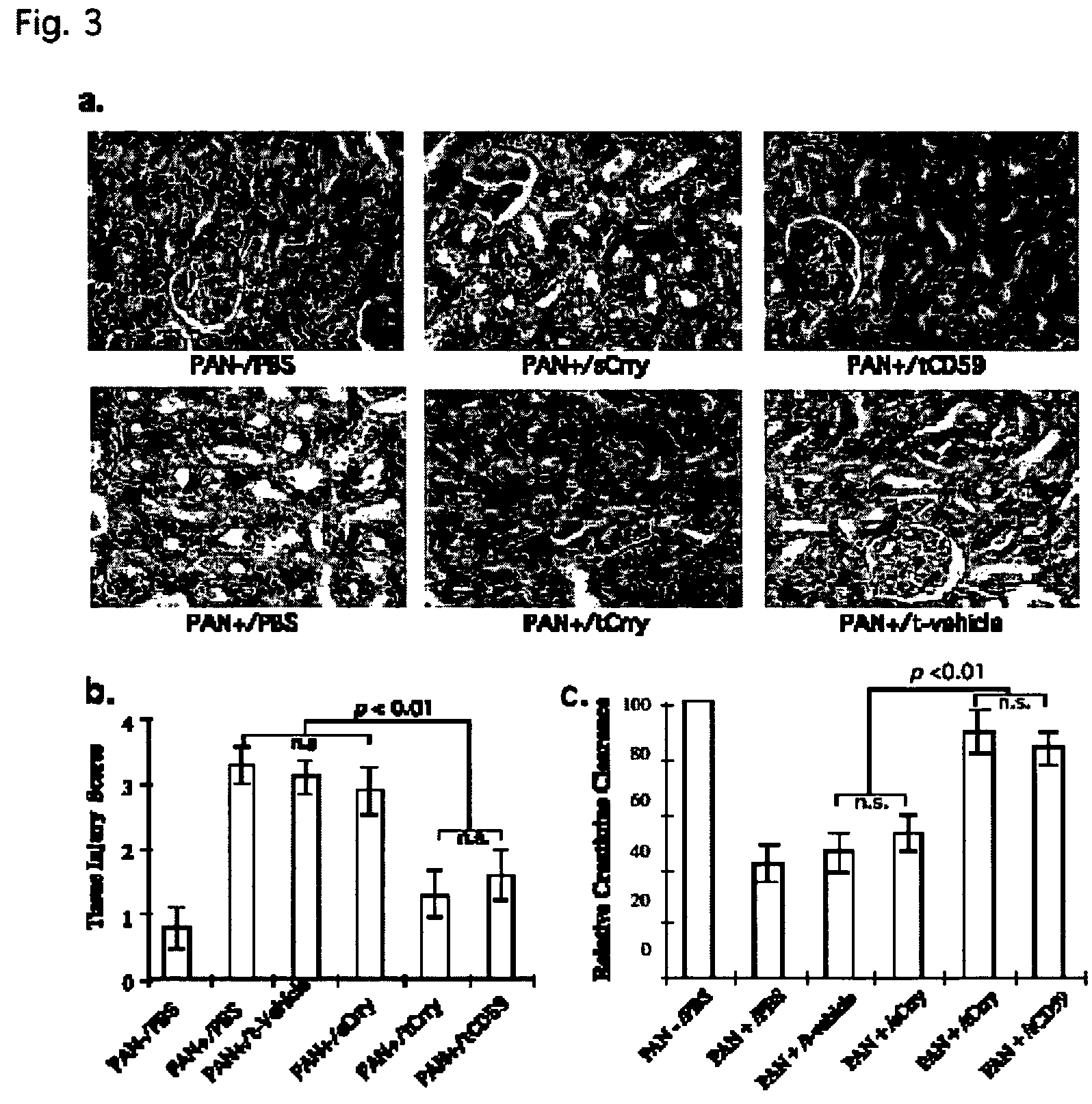
Systemic suppression of the complement system has been shown to be effective to treat inflammatory disease, yet at the potential cost of compromising host defense and immune homeostasis. Herein disclosed are methods for antigen-specific targeting of complement inhibitors that show that complement inhibitors targeted to the proximal tubular epithelium protect against tubulointerstitial injury and renal dysfunction in a rat model of nephrosis. It is shown that appropriate targeting of a systemically administered complement inhibitor to a site of disease markedy enhances efficacy and obviates the need to systemically inhibit complement. Additionally, it is shown by specifically inhibiting the terminal pathway of complement, that the membrane attack complex (MAC) plays a key role in proteinuria-induced tubulointerstitial injury, thus establishing the MAC as a valid target for pharmacological intervention in proteinuric disorders. The disclosed are compositions can be used in methods of treating pathogenic diseases and inflammatory conditions by modulating the complement system.
Owner:UNIVERSITY OF CHICAGO +1
Prescription zero: a non-pharmaceutical prescription device for prescribing, administering, monitoring, measuring and motivating a therapeutic lifestyle regimen for prevention and treatment of chronic diseases
InactiveUS8303500B2Good for healthAlter and maintain healthy lifestyleFinancePerson identificationRegimenPharmacological interventions
The invention discloses a wearable / handheld personal communication device with hardware and software sensor modules that sense and analyze all caregiver prescribed / monitored user-lifestyle activities, and deploys such analysis in improving user's overall health in terms of reduced risks for all-cause morbidities / mortalities and eventually a life without drugs. Termed Rx Zero, such method may be prescribed not just for maintaining a healthy lifestyle, but for treatment of chronic diseases with intent to wean the patients to minimal or zero pharmacological intervention, or in combination with medications to improve prognosis of the disease under treatment.The benefits of the Rx Zero method of the present invention extend not only to the individual and the community through high quality healthcare at lower cost, but payers by reducing the loss ratio on account of reduced cost of medical claims, and to the caregivers in terms of an effective tool that disseminates, implements and redefines “Primary Health Care” and “Prevention” at levels beyond the terms' currently understood scope that has transformed healthcare to sickcare.
Owner:RAHEMAN FAZAL
Livestock certification apparatus and method
A system for certification of animals, owners, attendants, venues, enterprises, and events relies on identification, traceability, and evaluation against selected criteria. Data maintained during agricultural production includes production, raising, selling, holding, treating, harvesting products like milk or wool, slaughter, transfer of ownership or venue, and veterinary and pharmacological interventions, whether medicaments, preventives, protocols, treatments, or inspections administered. Record keeping and selection, analysis, and notifications are according to criteria (particularly risks, exposures, or both) specified by any entity requesting or requiring a certification of a condition or lack thereof. Warnings may be triggered and distributed timely. Mobile units avoid traditional deficiencies in data, analysis, risk assessment, and timeliness.
Owner:TENG CHIA CHI
Tissue targeted complement modulators
InactiveUS8454963B2Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsTissue targetingEpithelium
Systemic suppression of the complement system has been shown to be effective to treat inflammatory disease, yet at the potential cost of compromising host defense and immune homeostasis. Herein disclosed are methods for antigen-specific targeting of complement inhibitors that show that complement inhibitors targeted to the proximal tubular epithelium protect against tubulointerstitial injury and renal dysfunction in a rat model of nephrosis. It is shown that appropriate targeting of a systemically administered complement inhibitor to a site of disease markedy enhances efficacy and obviates the need to systemically inhibit complement. Additionally, it is shown by specifically inhibiting the terminal pathway of complement, that the membrane attack complex (MAC) plays a key role in proteinuria-induced tubulointerstitial injury, thus establishing the MAC as a valid target for pharmacological intervention in proteinuric disorders. The disclosed are compositions can be used in methods of treating pathogenic diseases and inflammatory conditions by modulating the complement system.
Owner:UNIVERSITY OF CHICAGO +1
Methods for regulating inflammatory mediators and peptides useful therein
InactiveUS7544772B2Reduce releaseNervous disorderPeptide/protein ingredientsIntracellular signallingMANS peptide
Owner:BIOMARK PHARMACEUTICALS LTD +1
Feed for inducing high-level expression of reactive protein C of animal and application thereof
InactiveCN104489383AEasy to transportEasy to storeAnimal feeding stuffBiotechnologyDiabetes mellitus
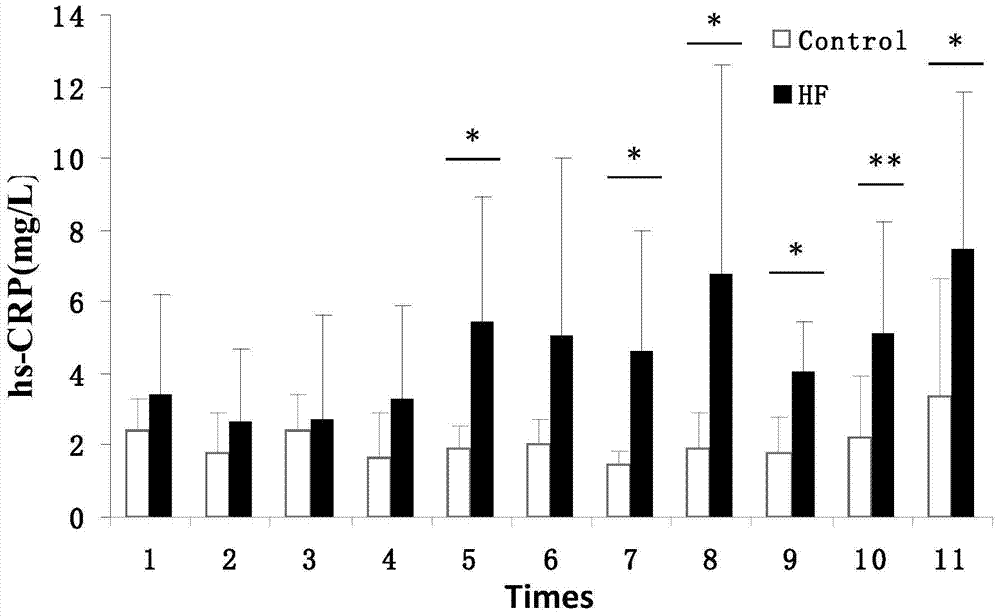
The invention discloses a feed for inducing high-level expression of a reactive protein C of an animal and application thereof. The feed for inducing high-level expression of the reactive protein C of the animal comprises the following components in percentage by mass: 10-20% of leaf lard, 1-2% of cholesterol and the balance of a basal feed in growing period, totaling 100%. Researches on high expression of CRP of machin diet-induced by the feed for inducing high-level expression of the reactive protein C of the animal have related important research significance and application value. The feed further has important actual meaning on research of inflammatory diseases and mechanism research of inflammatory diseases such as atherosclerosis, cardiovascular disease and even diabetes and arthritis, research on early warning and efficacy evaluation after pharmacological intervention. The raw materials of the feed for inducing high-level expression of the reactive protein C of the animal disclosed by the invention are low in cost, wide in source, easily available and good in stability, and the feed for inducing high-level expression of the reactive protein C of the animal is simple in preparation method, easy to convey and store and has relatively good application and popularization prospects.
Owner:GUANGDONG INST OF APPLIED BIOLOGICAL RESOURCES
Applications of dopamine I receptor agonists in preparing tumor treatment medicines
InactiveCN106924735AAntineoplastic agentsHeterocyclic compound active ingredientsBenign tumoursPharmacological interventions
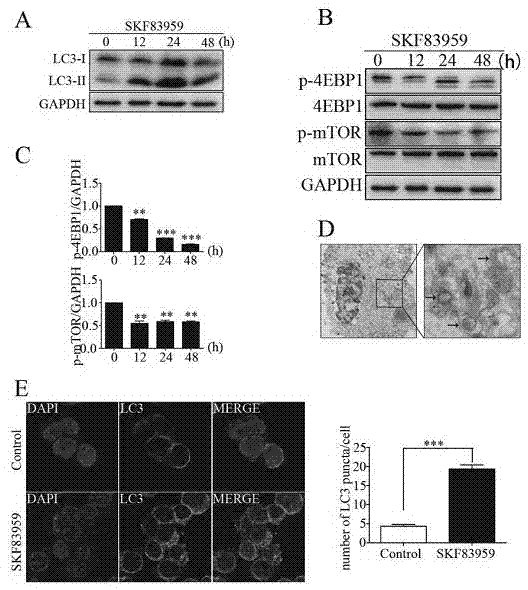
The invention belongs to the technical field of biological medicines, relates to novel medicinal applications of dopamine I receptor agonists, and in particular relates to novel applications of the dopamine I receptor agonists in preparing tumor treatment medicines. The dopamine I receptor agonists comprise DRD1 and DRD5 agonists, and are selected from SKF83959 or SKF38393 compounds. The cell culture and drug intervention experiments prove that the dopamine receptor agonist SKF83959 can inhibit the mTOR pathway for inducing autophagy, and increase ROS for prompting the cell death from the in-vitro and in-vivo levels, so that the growth of benign or malignant tumor can be inhibited. The dopamine I receptor agonists SKF83959 and SKF38393 can be used for preparing the medicines for treating tumors, wherein the tumors comprise the benign tumour including pituitary adenoma and the malignant tumors including malignant glioma, colon cancer, liver cancer, stomach cancer and the like.
Owner:RUIJIN HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Analytical method for beta-amyloid protein pathology by using thioflavine T staining
InactiveCN103278626AClear etiologyStrong impetusFluorescence/phosphorescencePharmacological interventionsDisease patient
The invention relates to an analytical method for beta-amyloid protein pathology by using thioflavine T staining. The method comprises the following processes: A, a patient pathological specimen source, B, an experimental animal pathological specimen source, C, treatment of patient and experimental animal brain tissue specimens, D, immumohistochemical staining of specimen beta-amyloid protein 1-42, E, thioflavine T staining of the specimen, and F, optical / fluorescence microscope pathological examination. The method has the advantages of introducing and widely using the thioflavine T to mark an amyloid protein disease patient brain tissue pathological specimen and assess the pharmacological intervention effect of alzheimer disease (AD) transgenic animals; compared with the traditional silver staining method and A beta immunohistochemical method, the method provided by the invention has the characteristics of being simple and feasible, and sensitive and reliable, is beneficial for clearing the nosetiology of cerebral hemorrhage, and has great guide value on formulation of patient secondary intervening measures. The wide application of the technology has a powerful promoting effect on clinical pathological research and translational medicine research of the A beta related diseases (alzheimer disease and amyloid angiopathy).
Owner:徐俊
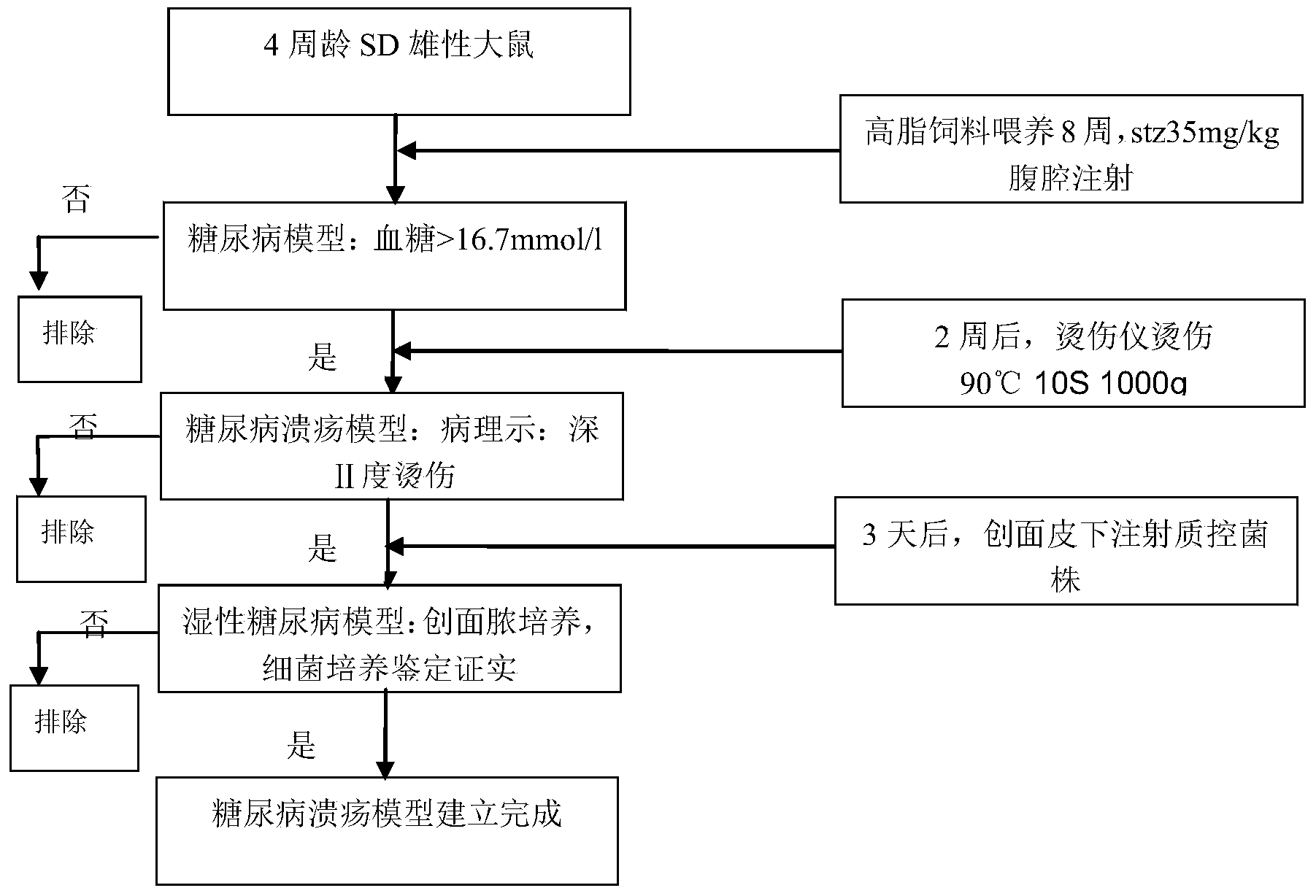
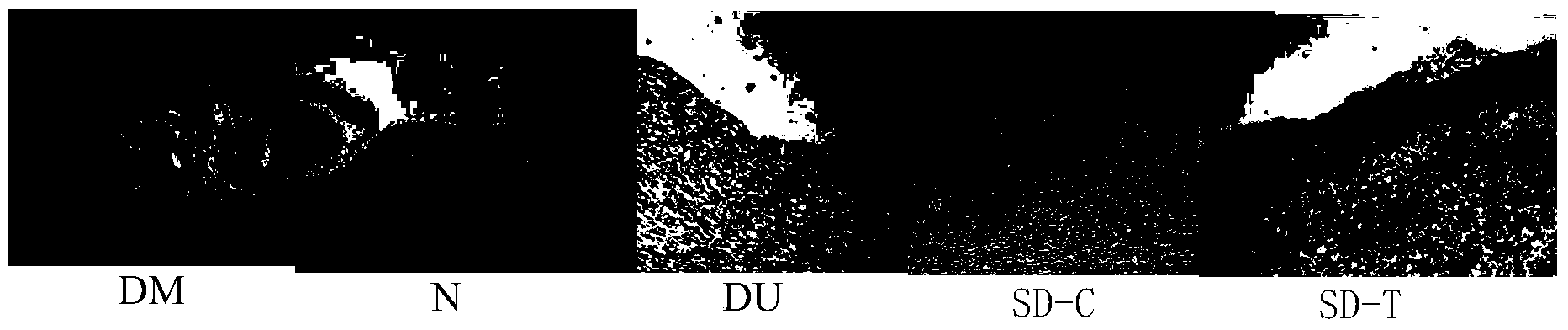
Method and kit for establishing experimental model of diabetic foot ulcer big mouse infected by staphylococcus aureus
InactiveCN103479680ALonger wound healing timeOrganic active ingredientsBacteria material medical ingredientsStaphylococcus cohniiDiabetes model
The invention discloses a method for establishing an experimental model of a diabetic foot ulcer big mouse infected by staphylococcus aureus. The method includes the following steps that after the big mouse is fed on high-fat diet for eight weeks, streptozotocin is injected, and a diabetic model is manufactured. After the diabetic model is stabilized, II-degree scalding is performed on the skin of the big mouse, and after three days, subcutaneous injection of suspension liquid with staphylococcus aureus as the bacterial strain is performed in each ulcer position. In addition, the invention further discloses a kit for establishing the experimental model of the diabetic foot ulcer big mouse infected by the staphylococcus aureus, wherein the kit comprises the staphylococcus aureus suspension liquid. The diabetic foot ulcer experimental animal model detects variation of biochemical indexes after pharmacological intervention, and a result shows that the model has the infection features of diabetic foot ulcer and concurrent gram-positive bacteria. The model has the advantages of being short in ulcer wound surface healing time, sensitive to drug reaction and the like.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Methods of treating mixed dyslipidemia and hypertriglycertdemia
ActiveUS11446282B2Organic active ingredientsMetabolism disorderDyslipidemiaPharmacological interventions
The present invention relates to pharmacological interventions with pemafibrate for moderate or severe hypertriglyceridemia.
Owner:KOWA CO LTD
Methods for attenuating release of inflammatory mediators and peptides useful therein
The present invention includes methods of inhibiting or suppressing cellular secretory processes. More specifically the present invention relates to inhibiting or reducing the release of inflammatory mediators from inflammatory cells by inhibiting the mechanism associated with the release of inflammatory mediators from granules in inflammatory cells. In this regard, the present invention discloses an intracellular signaling mechanism that illustrates several novel intracellular targets for pharmacological intervention in disorders involving secretion of inflammatory mediators from vesicles in inflammatory cells. Peptide fragments and variants thereof of MANS peptide as disclosed in the present invention are useful in such methods.
Owner:BIOMARK PHARMACEUTICALS LTD
Multifunctional blood substitute
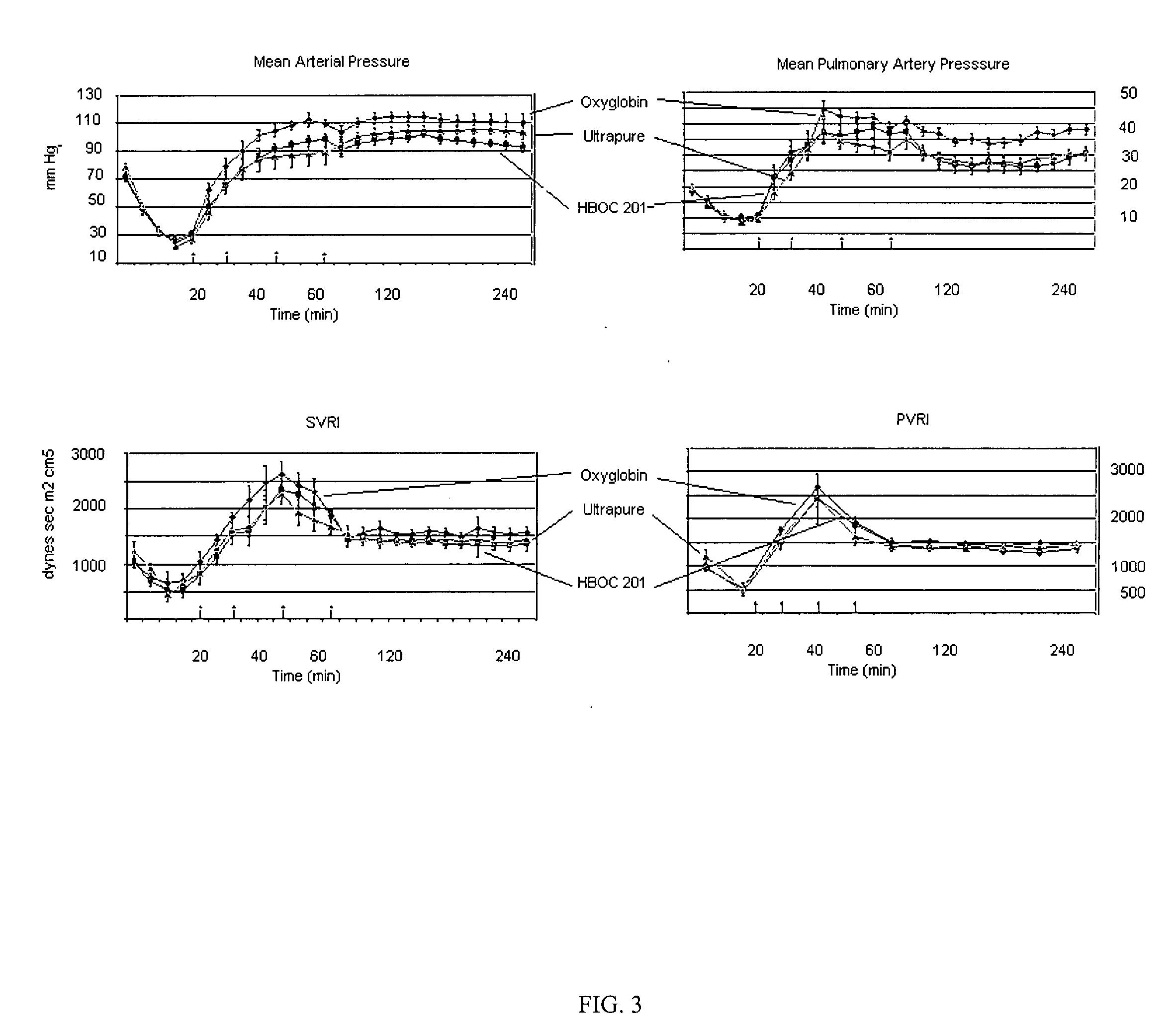
InactiveUS20070265195A1Peptide/protein ingredientsDead animal preservationPharmacological interventionsNormal blood volume
A pharmaceutical formulation capable of supplying replacement blood volume and tissue oxygenation as well as other functions, such as procoagulation and pharmacological interventions, in order to enhance survivability in patients with severe blood loss. The invention also discloses a formulation and method of using the formulation in bridging severe blood loss using a multifunctional blood substitute in a prehospital setting.
Owner:FREILICH DANIEL A
Methods and materials for assessing and treating obesity
ActiveUS20210072259A1Organic active ingredientsPeptide/protein ingredientsPhysiologyPharmacological interventions
This document relates to methods and materials for assessing and / or treating obese mammals (e.g., obese humans). For example, methods and materials for using one or more interventions (e.g., one or more pharmacological interventions) to treat obesity and / or obesity-related comorbidities in a mammal (e.g., a human) identified as being likely to respond to a particular intervention (e.g., a pharmacological intervention) are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Application of vitamin K1 fat emulsion injection
The invention relates to application of a fat emulsion injection using a vitamin K1. Particularly, the invention relates to an emulsion composition comprising the vitamin K1, soybean oil, phospholipid, glycerin and water. The emulsion composition is prepared by processes of preparation of a aqueous phase, preparation of an oil phase, preparation of a coarse emulsion, high-pressure emulsification,hot press sterilization and the like. The invention further relates to a method for preparing the emulsion composition and pharmaceutical application of the emulsion composition. The emulsion injection is used for insufficiency of the vitamin K or coagulation disorder diseases caused by dyssynthesis of coagulation factors II, VII, IX and X, which are generated due to pharmacological intervention interfering the activity of the vitamin K. The emulsion composition disclosed by the invention shows one or various technical effects as shown in the specification.
Owner:西安安健药业有限公司
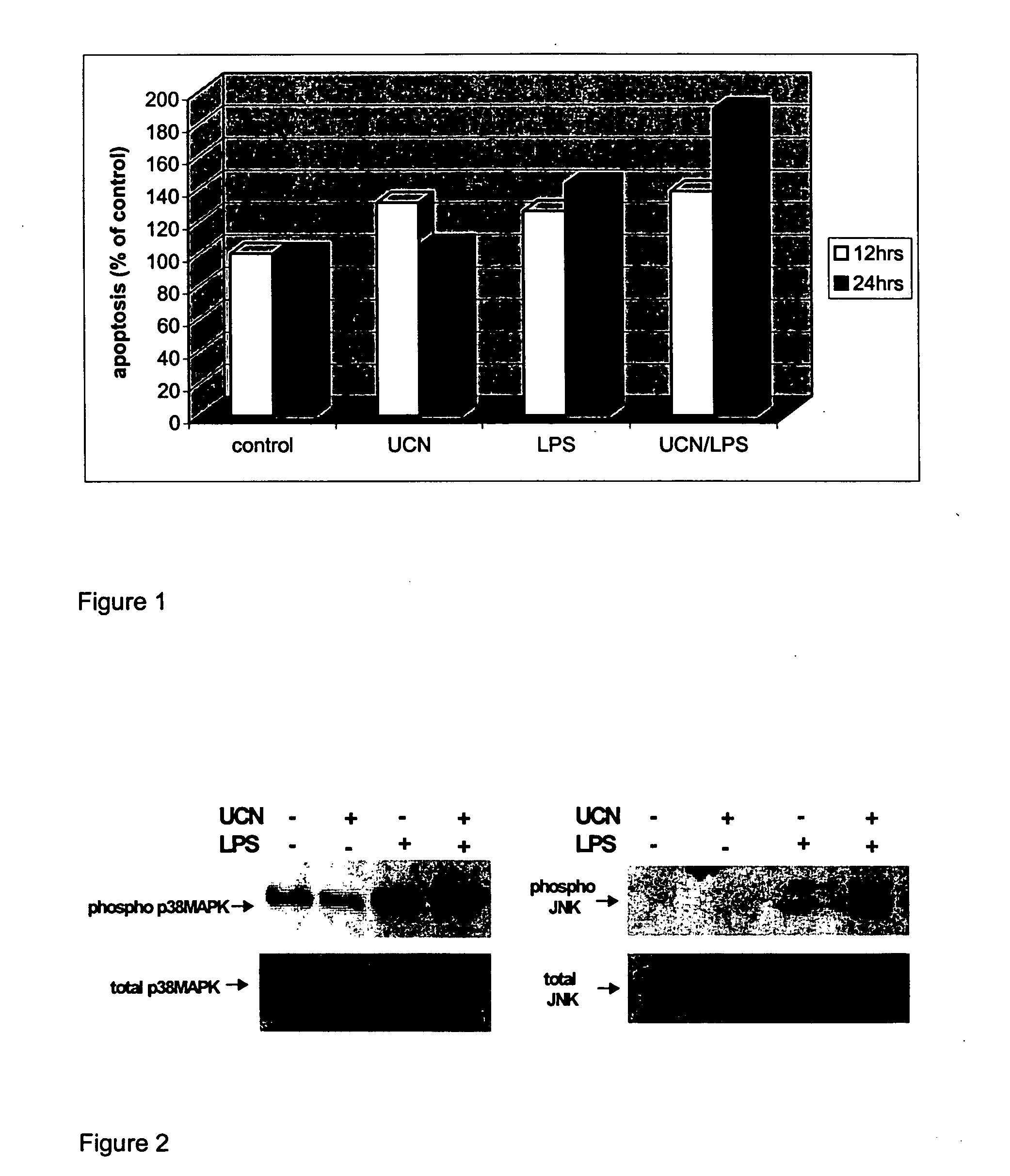
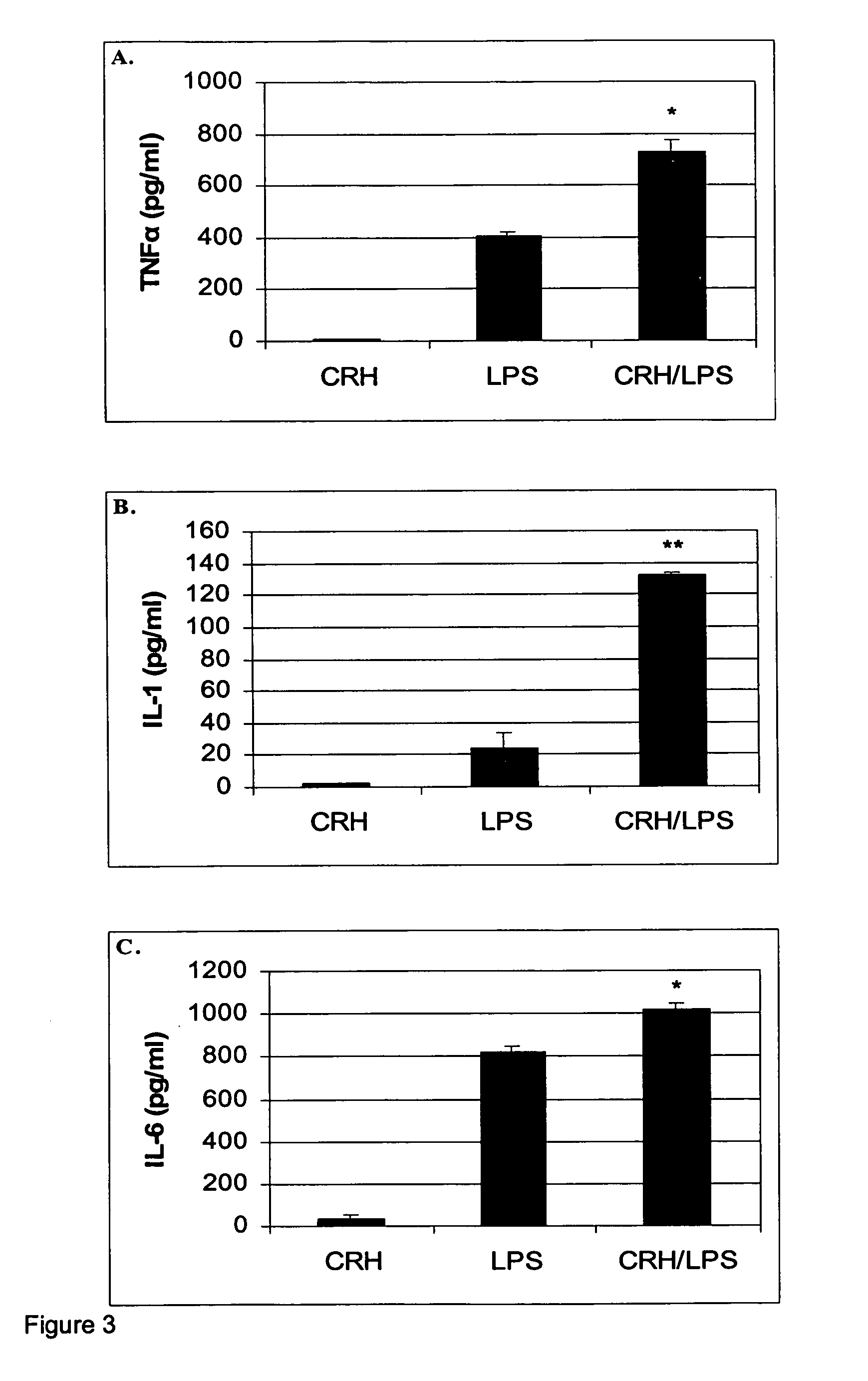
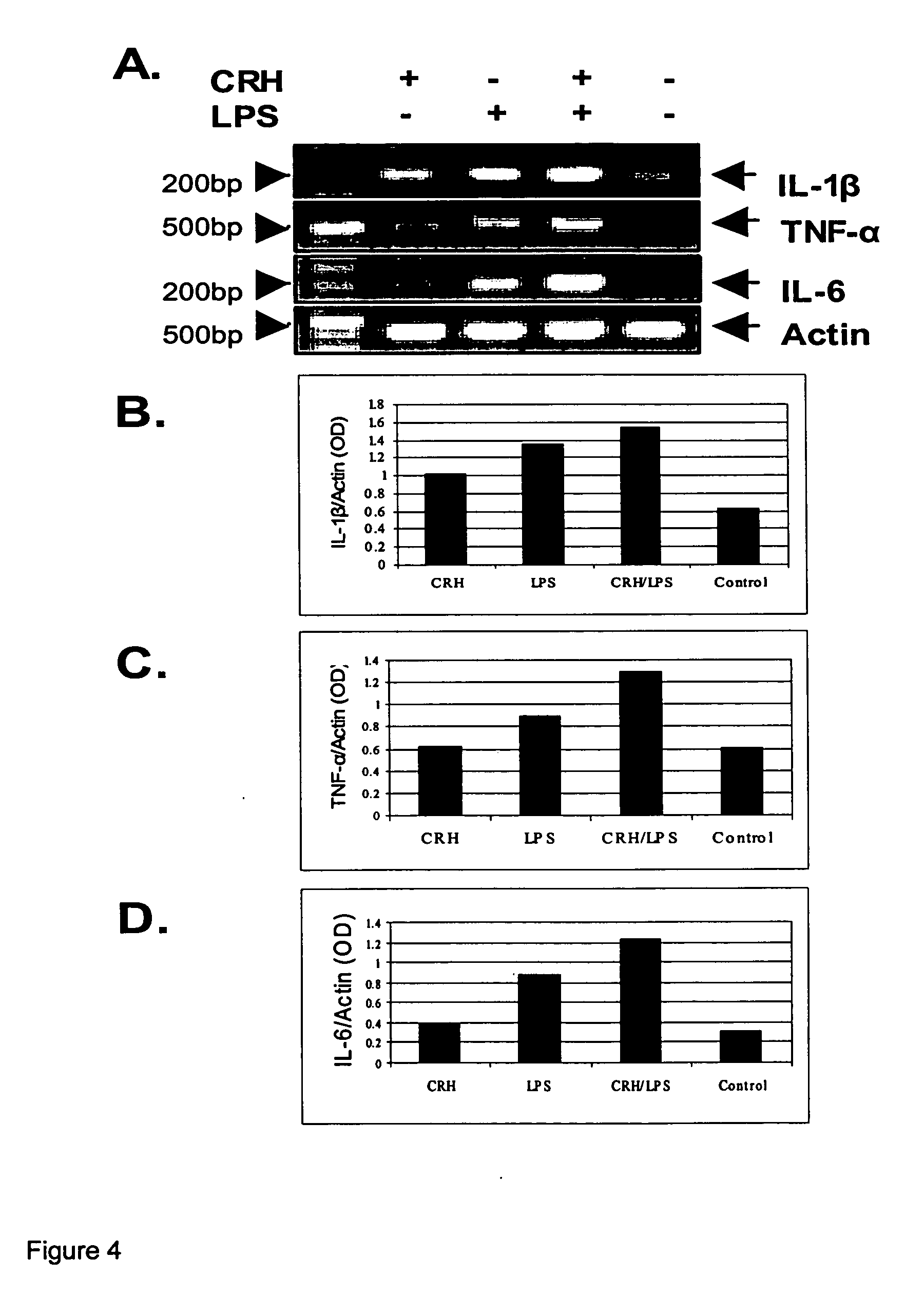
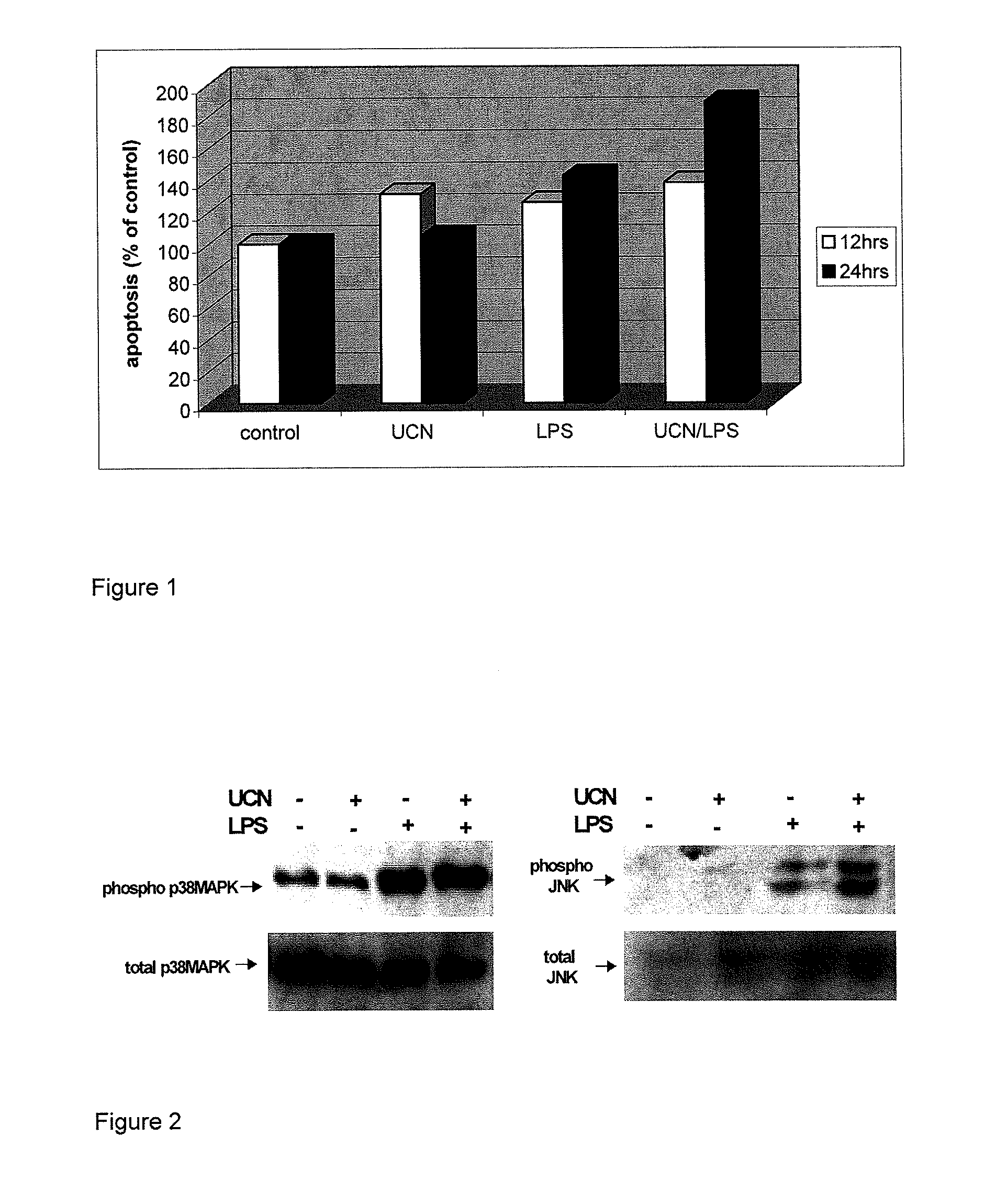
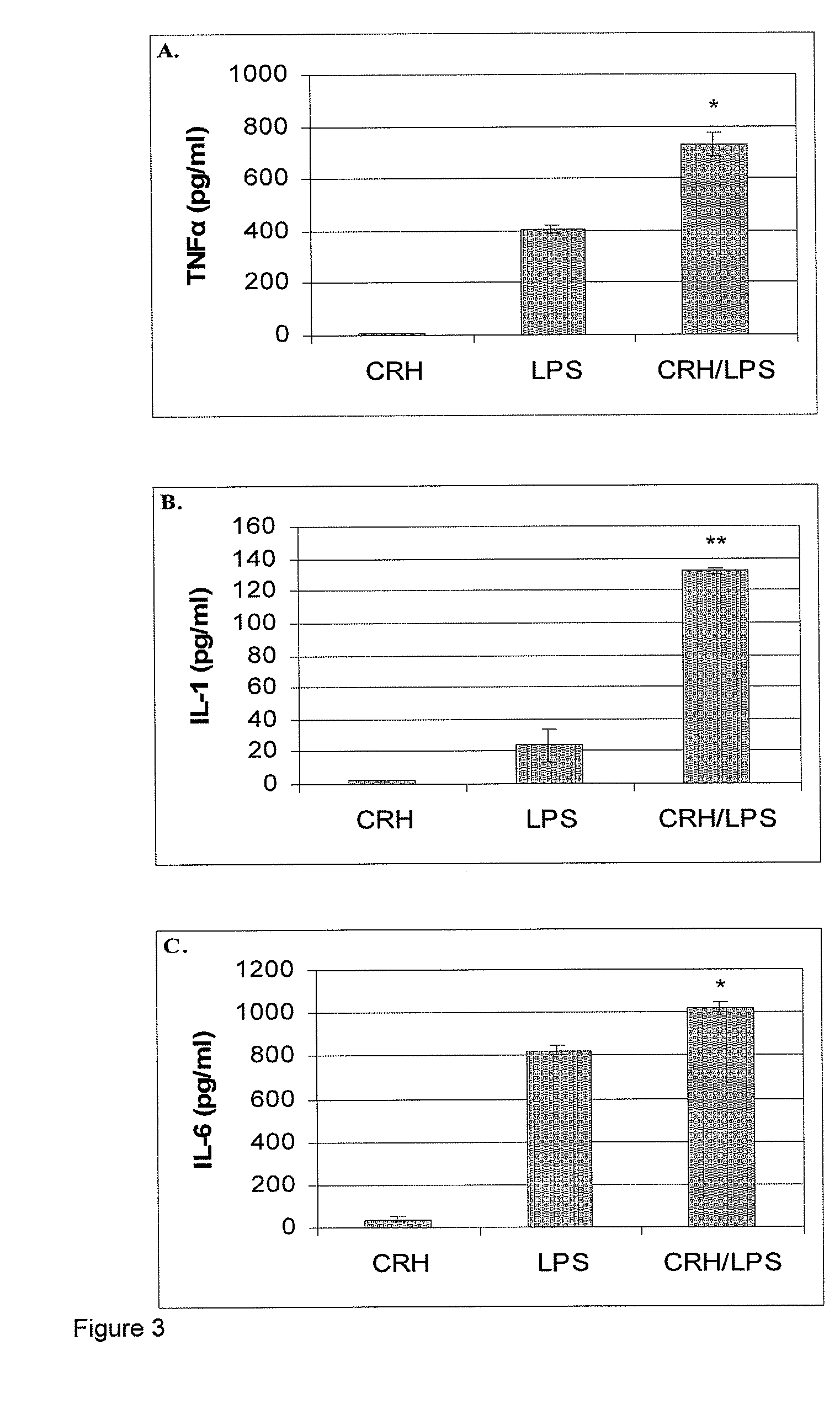
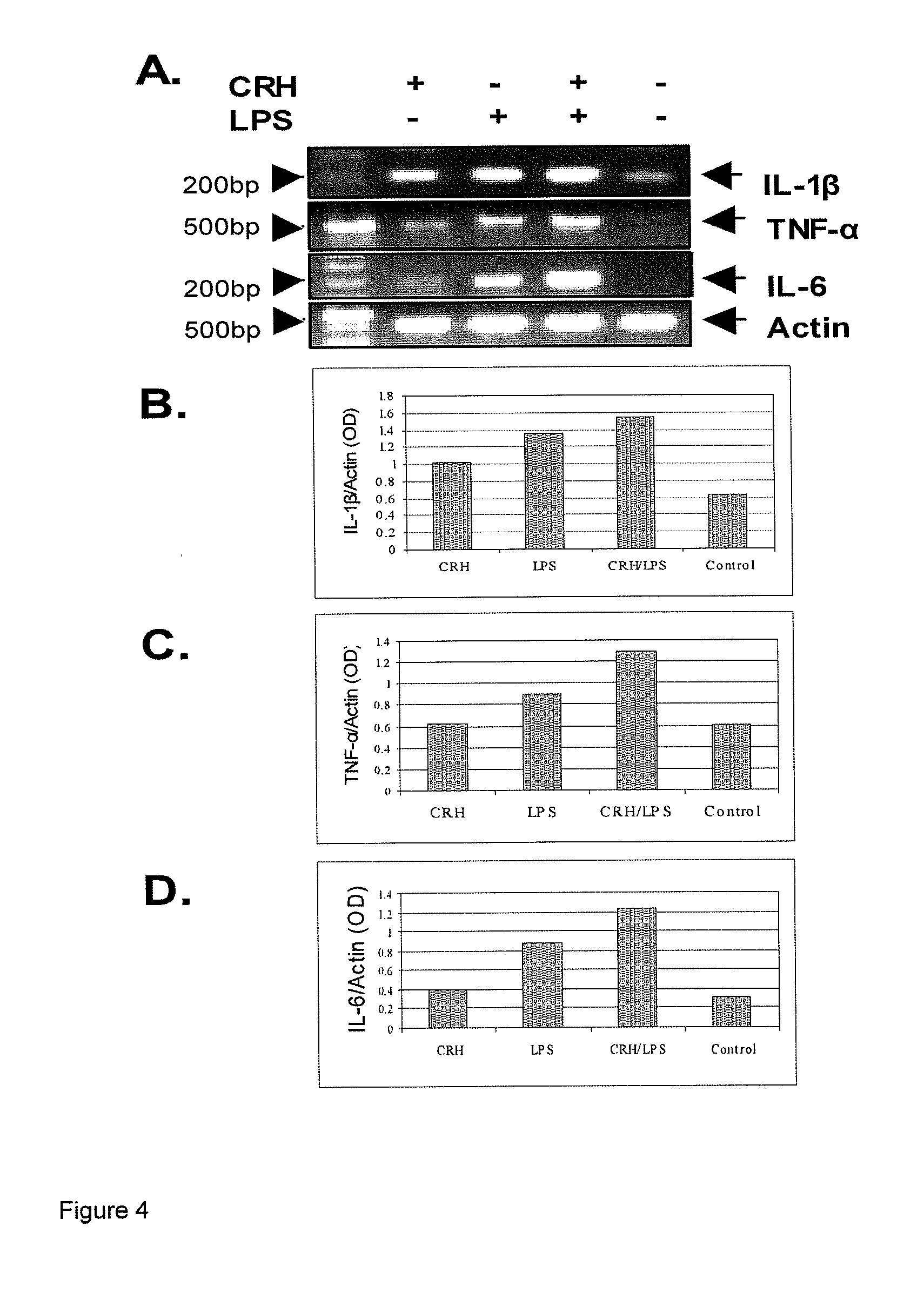
Use of the crh (corticotropin releasing hormone)-ucn (urocortin) system in the treatment of inflammatory diseases
InactiveUS20060135417A1Enhanced LPS-induced TNF-αInflammatory responseOrganic active ingredientsNervous disorderPharmacological interventionsApoptosis
The invention relates to the use of corticotropin-releasing hormone (CRH) receptor-1 (R1) antagonists and / or CRH-R2 receptor agonists for the treatment of inflammatory diseases via regulation of monocyte / macrophage cell activation, proliferation, differentiation, apoptosis, and inflammatory cytokine production. As CRH system we define natural and synthetic CRH and urocortin (UCN) agonists and antagonists for the CRH-R1 and CRH-R2 receptors and their subtypes as well as the CRH-binding protein (BP), a CRH pseudo-receptor. The invention is directed towards pharmacological intervention for the amelioration or treatment of inflammatory diseases using the CRH system-mediated control of monocyte / macrophage cells which play a key role in initiating and maintaining the inflammatory response via production of pro-inflammatory cytokines such as is the interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-alpha. By the term inflammation we define the response of an organism to noxious endogenous or exogenous stimuli causing tissue injury. Inflammation is a host defence mechanism, which might harm the defending organism. The invention also provides methods for the in vitro and in vivo evaluation of natural and synthetic CRH system modulators for the control of the monocyte / macrophage system.
Owner:BIONATURE E A LTD
Method and kit for establishing experimental model of diabetic foot ulcer big mouse infected by Escherichia coli
InactiveCN103479681AEase of evaluationGood repeatabilityOrganic active ingredientsBacteria material medical ingredientsEscherichia coliDiabetes model
The invention discloses a method for establishing an experimental model of a diabetic foot ulcer big mouse infected by Escherichia coli. The method includes the following steps that after the big mouse is fed on high-fat diet for eight weeks, streptozotocin is injected, and a diabetic model is manufactured. After the diabetic model is stabilized, II-degree scalding is performed on the skin of the big mouse, and after three days, subcutaneous injection of Escherichia coli suspension liquid is performed in each ulcer position. In addition, the invention further discloses a kit for establishing the experimental model of the diabetic foot ulcer big mouse infected by the Escherichia coli, wherein the kit comprises the Escherichia coli suspension liquid. The diabetic foot ulcer experimental animal model detects variation of biochemical indexes after pharmacological intervention, and a result shows that the model has the infection features of diabetic ulcer and concurrent Gram-negative bacteria. The model has the advantages of being short in ulcer wound surface healing time, sensitive to drug reaction and the like.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Vitamin K1 fat emulsion injection
ActiveCN109806225AOrganic active ingredientsEmulsion deliveryPharmacological interventionsPhospholipid
The invention relates to a vitamin K1 fat emulsion injection. Particularly, the emulsion composition comprises a vitamin K1, soybean oil, phospholipid, glycerin and water. The composition is preparedby processes of preparation of a aqueous phase, preparation of an oil phase, preparation of a coarse emulsion, high-pressure emulsification, hot press sterilization and the like. The invention furtherrelates to a method for preparing the emulsion composition and pharmaceutical application of the emulsion composition. The emulsion injection is used for insufficiency of the vitamin K or coagulationdisorder diseases caused by dyssynthesis of coagulation factors II, VII, IX and X, which are generated due to pharmacological intervention interfering the activity of the vitamin K. The emulsion composition disclosed by the invention shows one or various technical effects as shown in the specification. For example, the composition can be used according to a dose of 10mg each time, 1 to 2 times per day and a total dose of 40mg at most in 24 hours, and an administration dosage and frequency are regulated according to prothrombin time response or clinical symptoms; the using mode is intravenousadministration, infusion can directly adopt intravenous injection without dilution, or the vitamin K1 fat emulsion injection is subjected to intravenous dripping after being diluted with 5% glucose injection.
Owner:西安安健药业有限公司
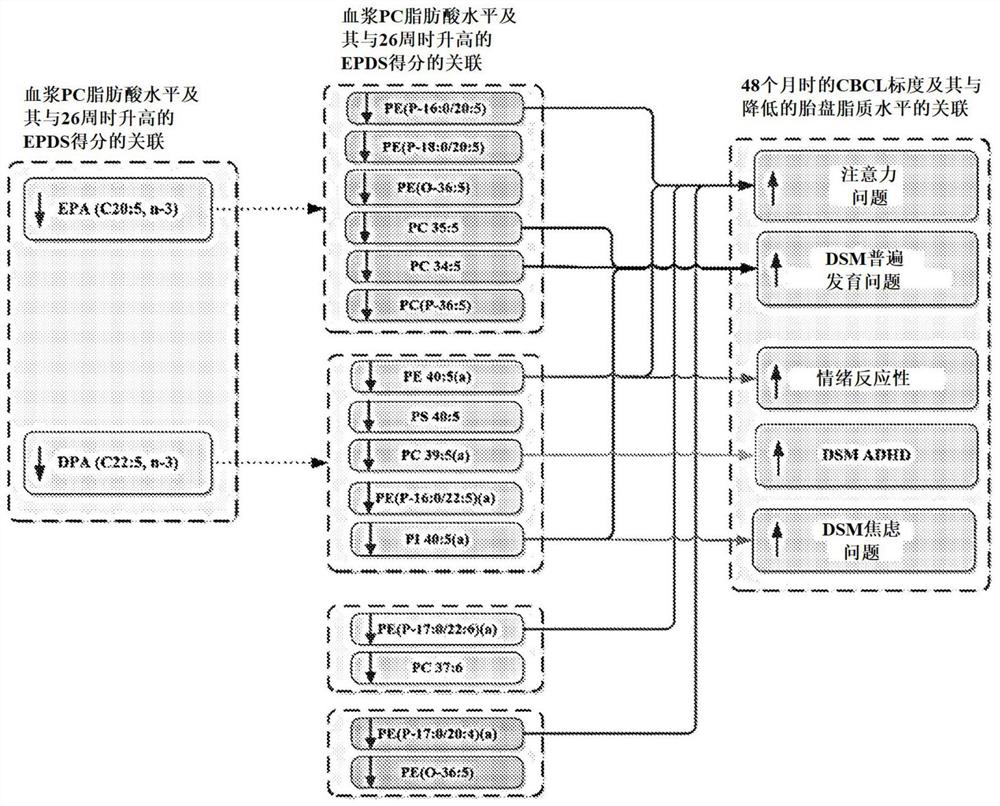
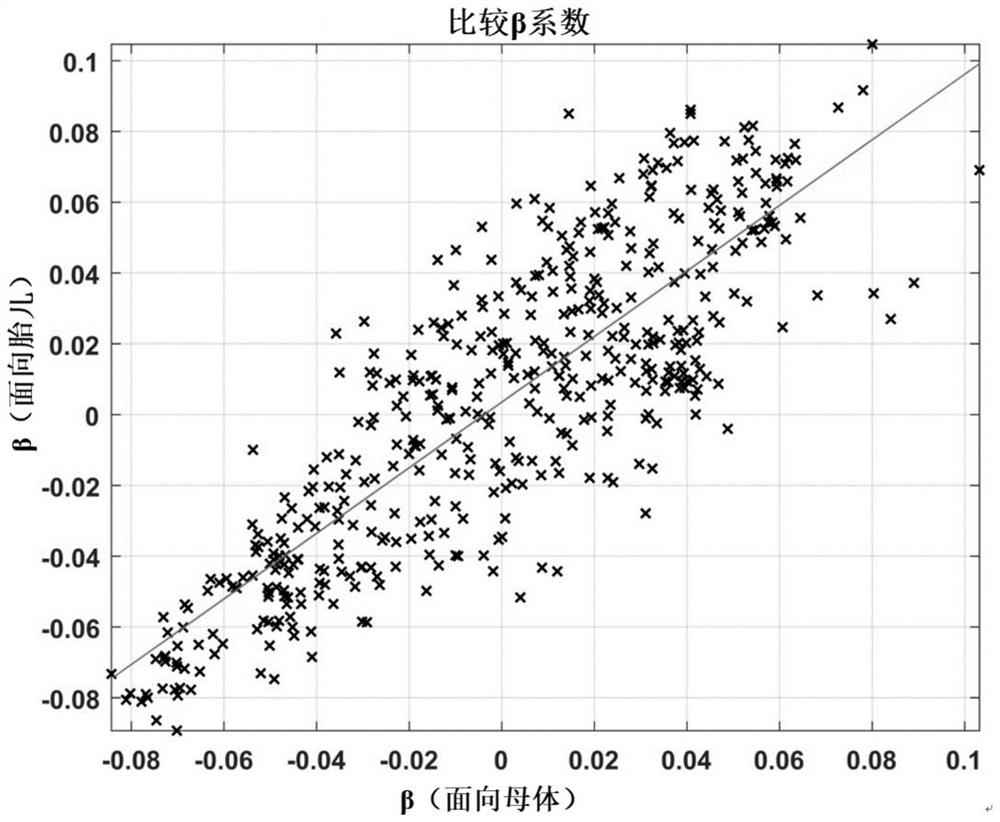
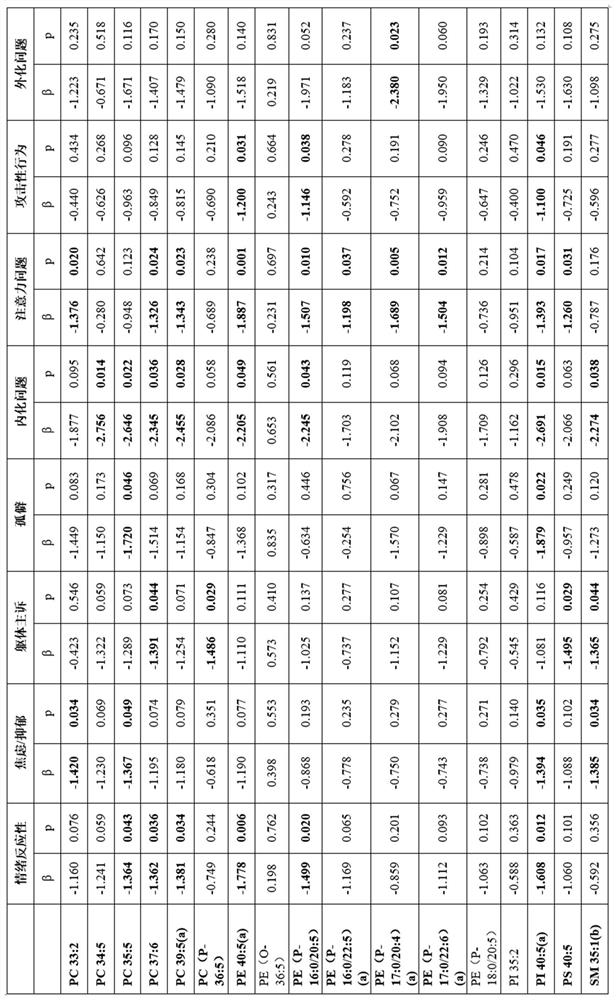
Maternal dpa for benefit of mother and/or child's mental health
This is the first ever report to uncover a tangible lipidomic basis by which maternal emotional well-being may influence fetal neuro-development and the later risk for psychopathology. This novel insight points to the potential utility of nutritional approaches among pregnant women with high levels of depressive symptoms in the prevention of offspring risk for later socio-emotional behavioral problems predictive of future psychopathology. Such a novel approach is particularly pertinent in light of the preference to avoid pharmacological interventions such as anti-depressant medications during pregnancy.
Owner:AGENCY FOR SCI TECH & RES +1
Methods of Treatment of Hypertriglyceridemia
InactiveUS20190224169A1Reduce plasma triglycerideAntibacterial agentsOrganic active ingredientsPharmacological interventionsSevere hypertriglyceridemia
The present invention relates to pharmacological interventions with pemafibrate for moderate or severe hypertriglyceridemia.
Owner:KOWA CO LTD
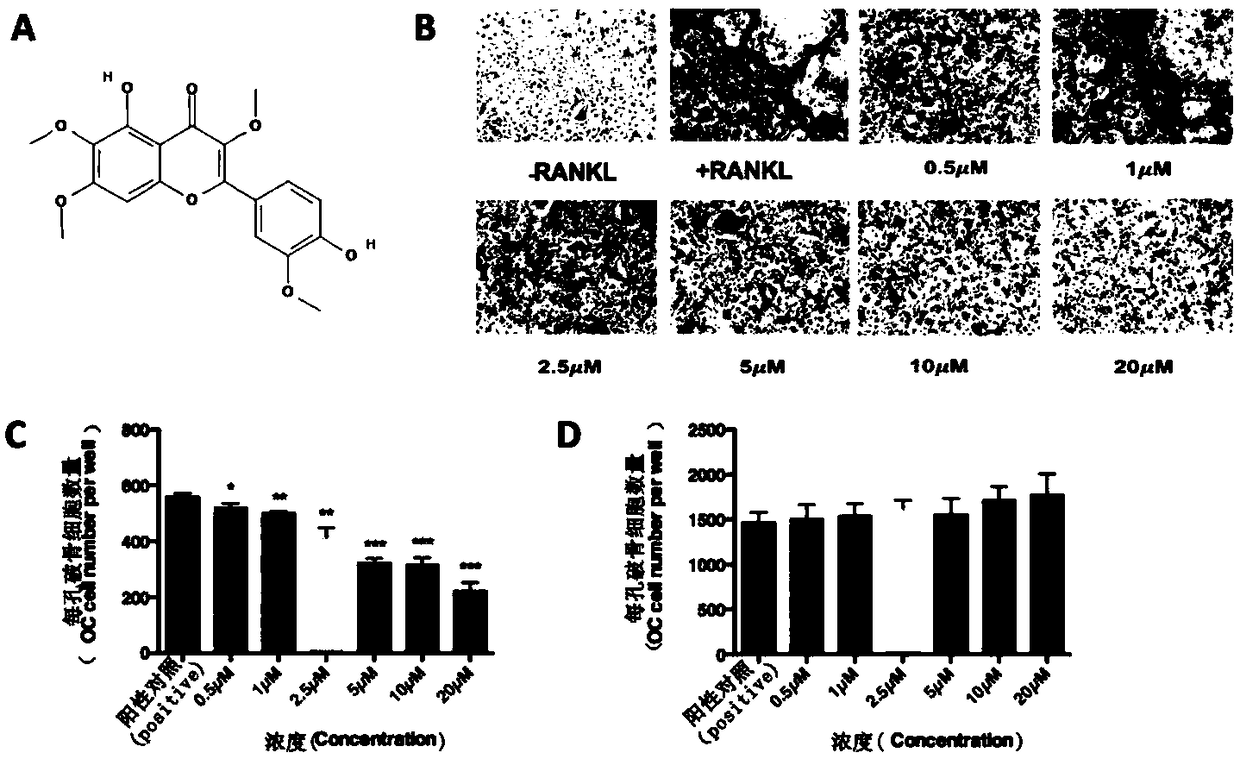
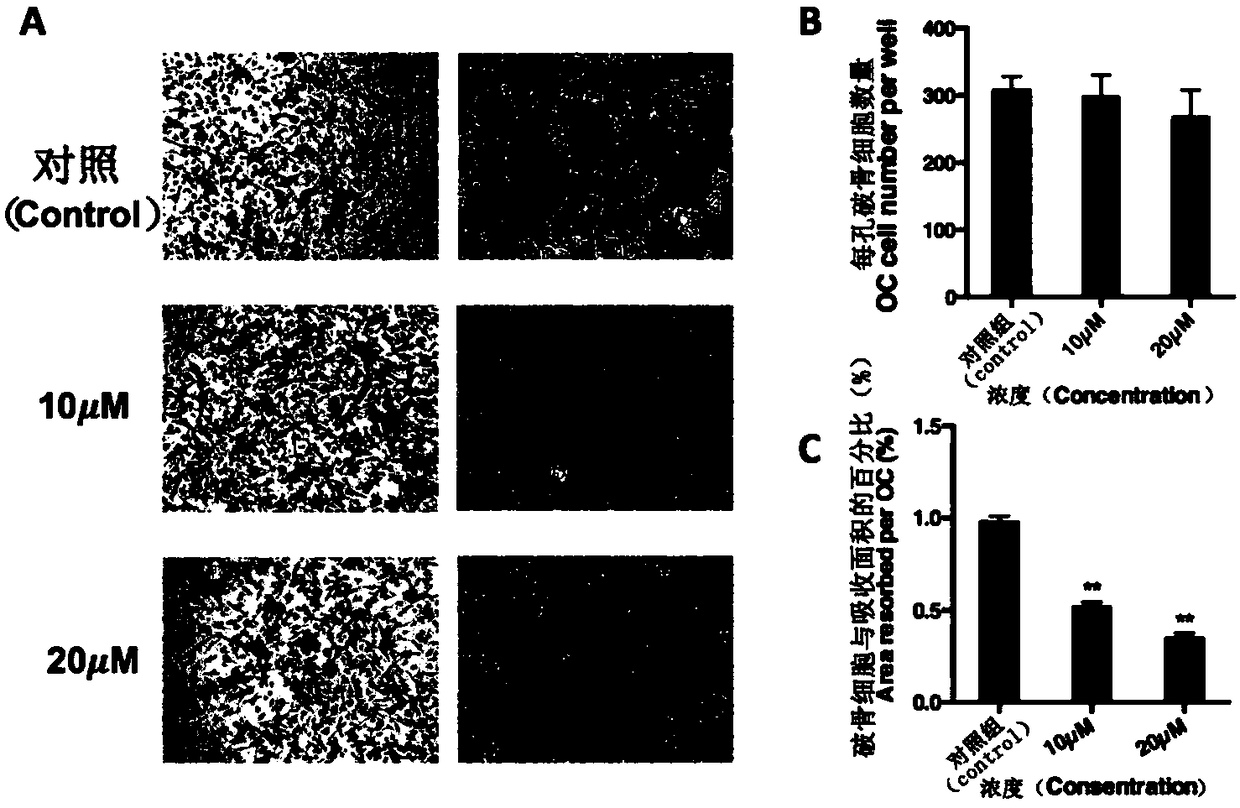
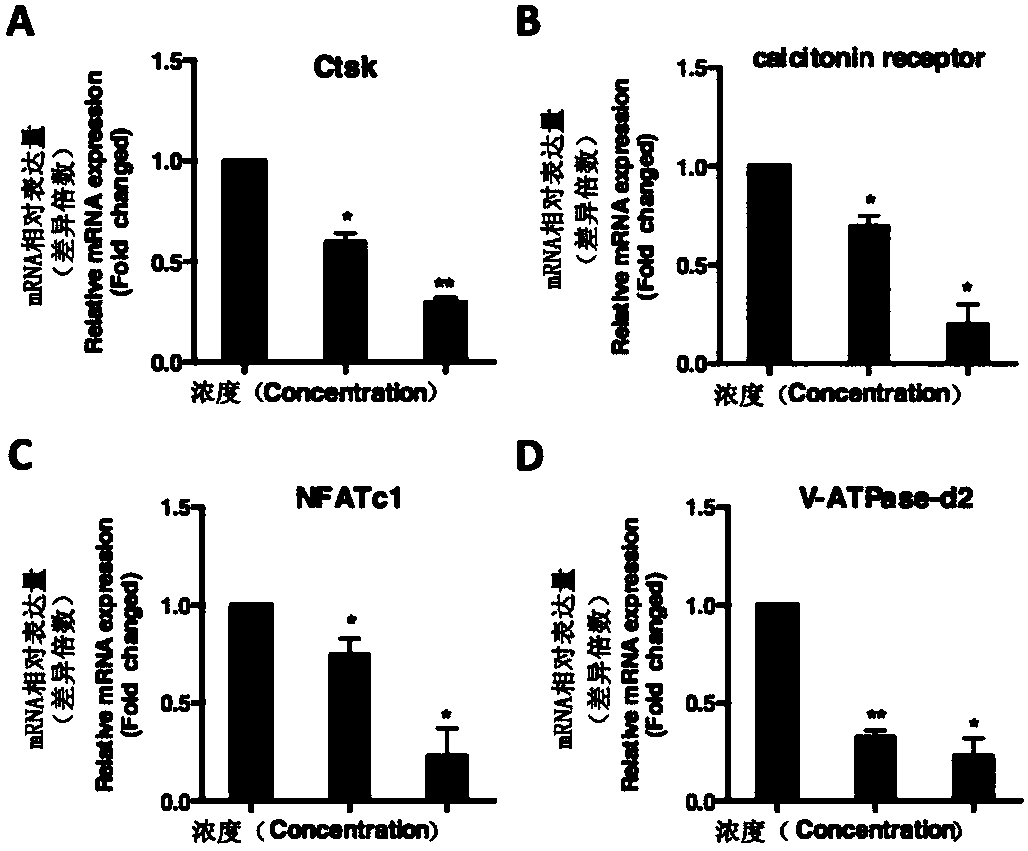
Application of chrysosplenetin to osteoporosis treatment
ActiveCN108078977AInhibition formationFacilitates two-way regulationOrganic active ingredientsSkeletal disorderOsteoblastNo-Observed-Effect Levels
The invention discloses an application of chrysosplenetin to osteoporosis treatment. The chrysosplenetin has the functions of simultaneously inhibiting osteoclast formation and promoting two-way regulation of osteoblast in osteoporosis, is low in no observed effect level and good in osteoporosis regulation effect. Besides, the chrysosplenetin has low gastrointestinal reaction and liver-kidney metabolic reaction. The intervention approach of chrysosplenetin-related medicines is clear. The application illuminates that a molecular mechanism in a bone microenvironment and a potential pharmacological intervention approach are of great importance for identifying new treatment compounds.
Owner:洪郭驹
Use of the crh (corticotropin releasing hormone) - ucn (urocortin) system in the treatment of inflammatory diseases
InactiveUS20090247558A1Enhanced LPS-induced TNF-αInflammatory responseOrganic active ingredientsBiocideDiseaseApoptosis
The invention relates to the use of corticotropin-releasing hormone (CRH) receptor-1 (R1) antagonists and / or CRH-R2 receptor agonists for the treatment of inflammatory diseases via regulation of monocyte / macrophage cell activation, proliferation, differentiation, apoptosis, and inflammatory cytokine production. As CRH system we define natural and synthetic CRH and urocortin (UCN) agonists and antagonists for the CRH-R1 and CRH-R2 receptors and their subtypes as well as the CRH-binding protein (BP), a CRH pseudo-receptor. The invention is directed towards pharmacological intervention for the amelioration or treatment of inflammatory diseases using the CRH system-mediated control of monocyte / macrophage cells which play a key role in initiating and maintaining the inflammatory response via production of pro-inflammatory cytokines such as is the interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-alpha. By the term inflammation we define the response of an organism to noxious endogenous or exogenous stimuli causing tissue injury. Inflammation is a host defence mechanism, which might harm the defending organism. The invention also provides methods for the in vitro and in vivo evaluation of natural and synthetic CRH system modulators for the control of the monocyte / macrophage system.
Owner:BIONATURE E A LTD
Methods of treating mixed dyslipidemia and hypertriglyceridemia
ActiveUS20200147051A1Organic active ingredientsMetabolism disorderDyslipidemiaPharmacological interventions
The present invention relates to pharmacological interventions with pemafibrate for moderate or severe hypertriglyceridemia.
Owner:KOWA CO LTD
Methods of preventing cardiovascular events in residual risk dyslipidemic populations
ActiveUS10864198B2Reduce riskPrevents the cardiovascular eventMetabolism disorderPharmaceutical delivery mechanismDyslipidemiaPharmacological interventions
The present invention provides pharmacological interventions for the treatment of dyslipidemia, and to the reduction of residual risk of cardiovascular disease and adverse cardiovascular events in patients on intense statin use or with well-controlled LDL-C concentrations. In particular, the invention relates to the use of pemafibrate to prevent cardiovascular events in populations at-risk due to risk factors such as type 2 diabetes mellitus with dyslipidemia in spite of intense statin use or well-controlled LDL-C.
Owner:KOWA CO LTD
A method for evaluating the antibacterial activity of Andrographis paniculata
ActiveCN103792259BStrong specificityIncreased sensitivityMaterial heat developmentPharmacological interventionsAntibacterial activity
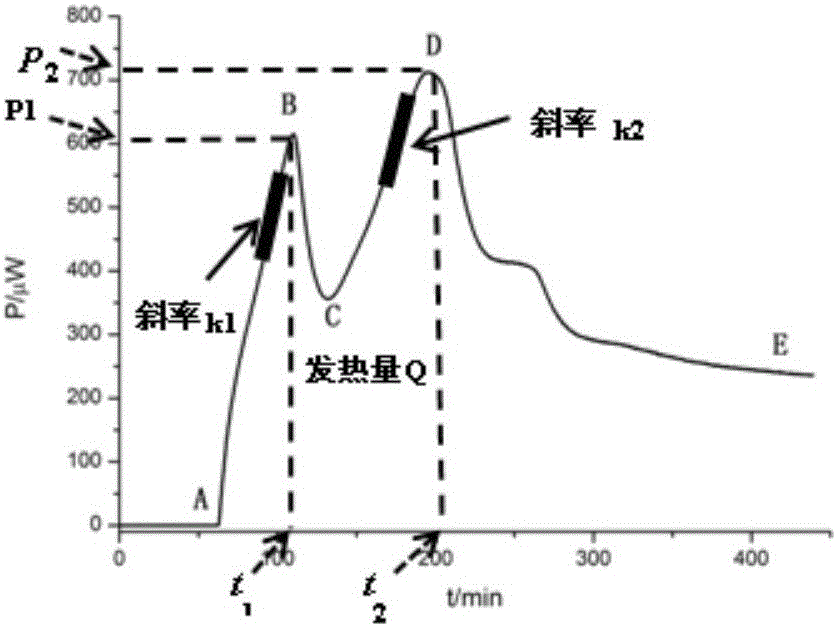
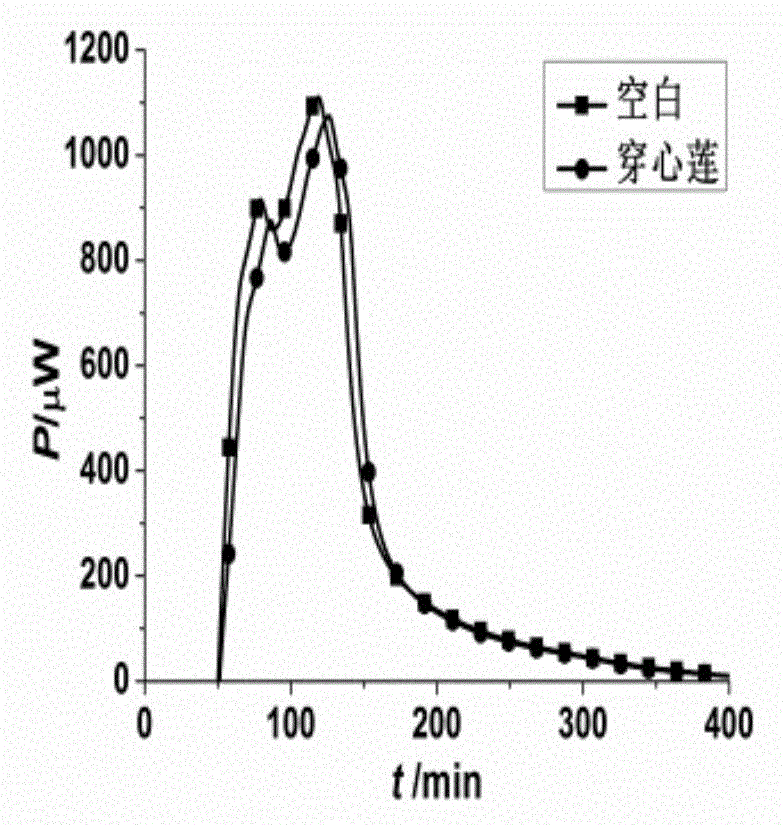
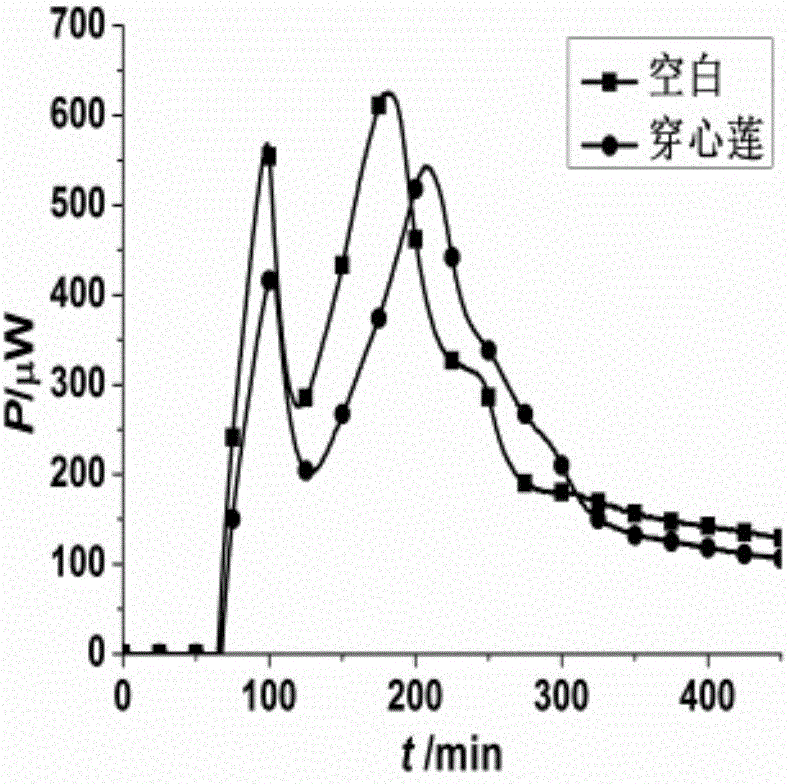
The invention discloses a method for evaluating antimicrobial activity of andrographis paniculata. According to the method, the antimicrobial activity of andrographis paniculata is evaluated by antibacterial rate I%, wherein the I% is calculated according to the following formula (1):I%=(t2-t0) / t0*100%(1), t0 refers to the time to peak of a second exponential growth phase in a growth and metabolism thermogram of bacteria which is not subjected to pharmacological intervention, t2 refers to the time to peak of a second exponential growth phase in a growth and metabolism thermogram of bacteria which is subjected to intervention of andrographis paniculata, and the concentration of a test solution of the andrographis paniculata interfering growth and metabolism of the bacteria ranges from 0.4mg / mL to 4 mg / mL, preferably 3mg / mL. The method provided by the invention can be used for qualitatively analyzing the antimicrobial activity of andrographis paniculata produced in different production areas, different preparation methods, different production batches or different growth time, and the like.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
A kind of pharmaceutical composition for treating spinal cord injury and repairing and application thereof
ActiveCN114522236BValidate potential interactionsReduce cutting actionCompounds screening/testingOrganic active ingredientsTissue repairLysosome
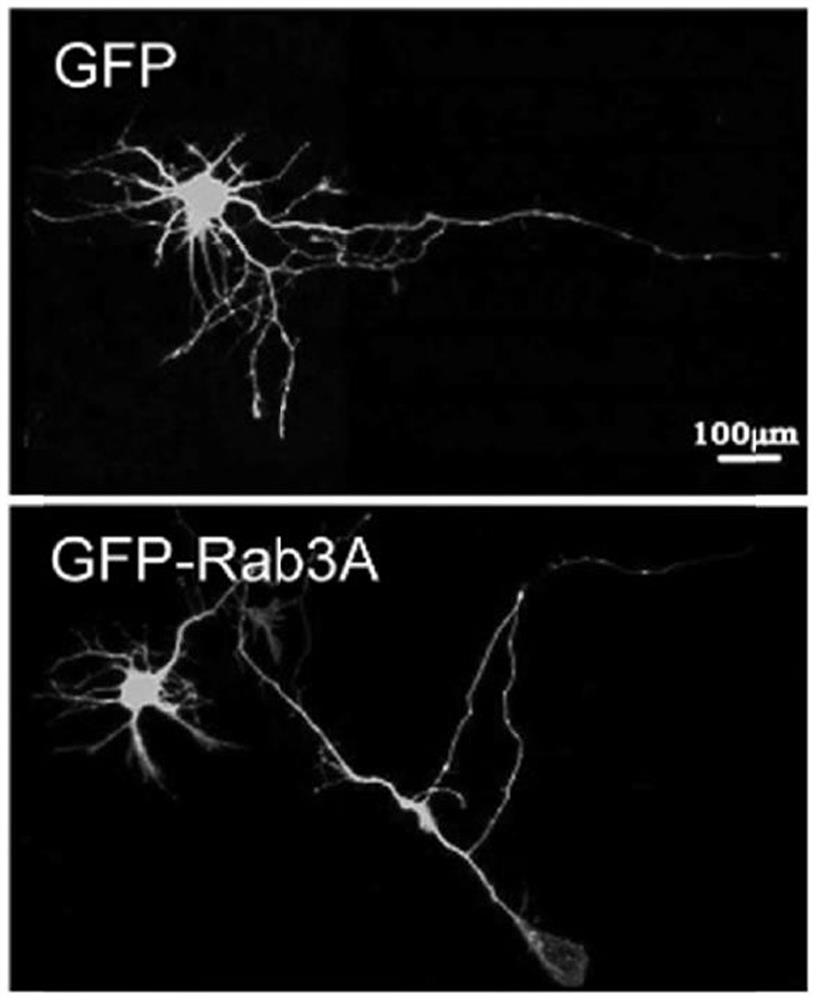
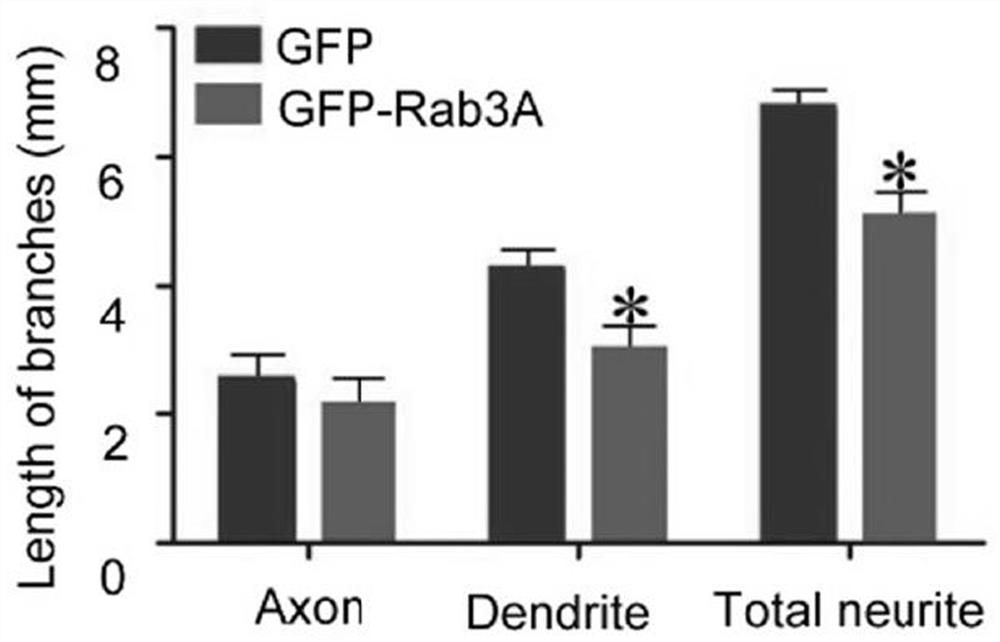
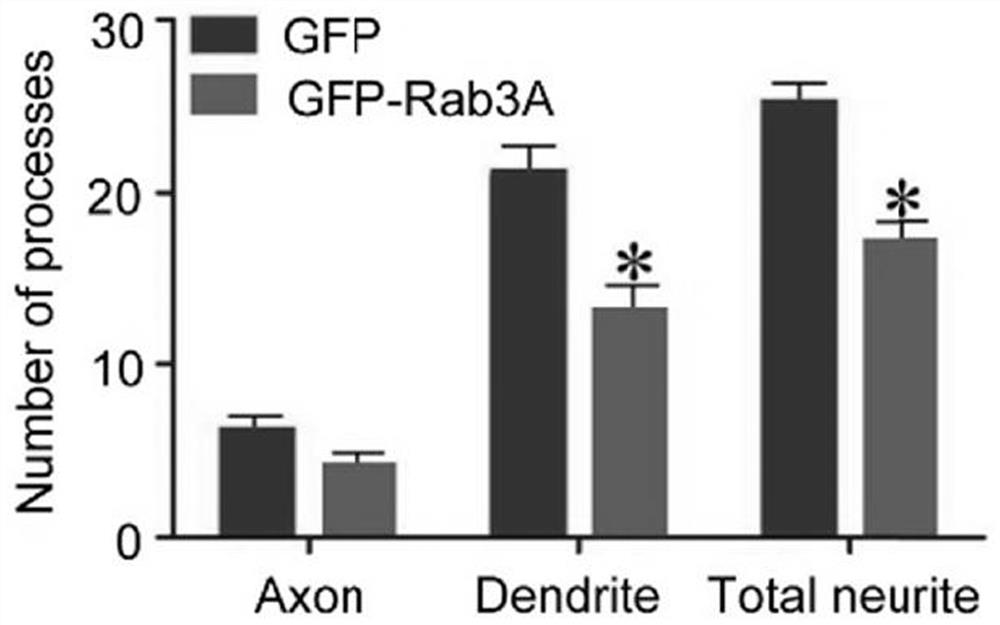
The present invention relates to a pharmaceutical composition for treating and repairing spinal cord injury and its application. The present invention finds through research that Rab3A plays a key role in SCI, and interacts with Spastin to regulate neurite outgrowth. By identifying proteins that were differentially expressed in SCI, it was demonstrated that Rab3A expression levels were downregulated during SCI. In addition, it was also found that Rab3A can physically interact with Spastin and regulate Spastin degradation through the lysosomal pathway, thereby affecting Spastin function. Collectively, these findings highlight a signal transduction pathway in which Rab3A-mediated spastin degradation regulates neurite branch formation and growth. Since SCI is a major cause of disability, repairing the structural defects of the spinal cord caused by injury or degeneration is crucial in the modern field of regenerative medicine. A variety of pharmacological interventions can be designed to treat SCI and assist with associated tissue repair, and can be combined with other cellular interventions.
Owner:JINAN UNIVERSITY
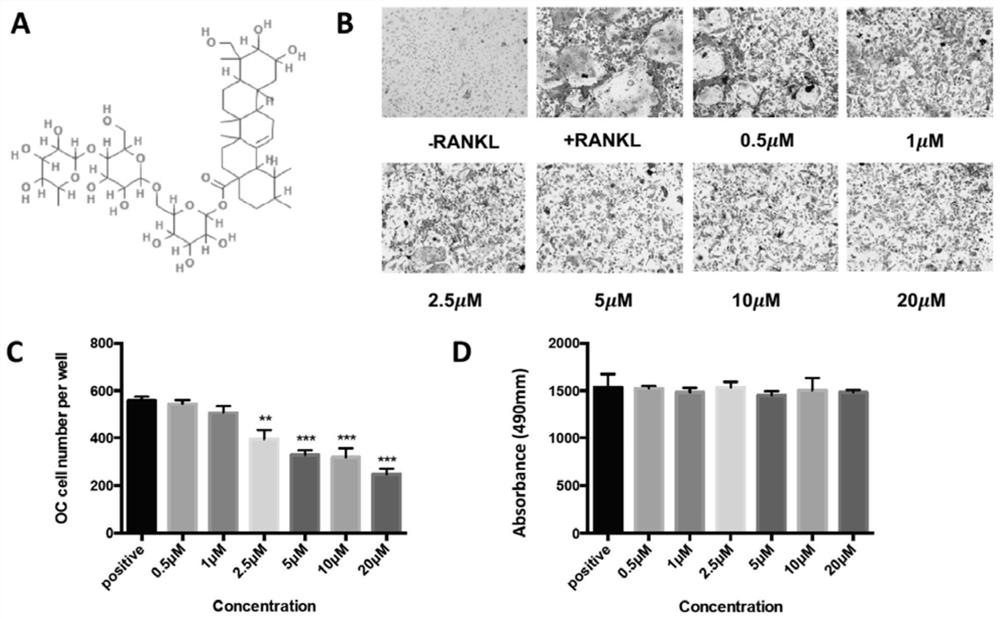
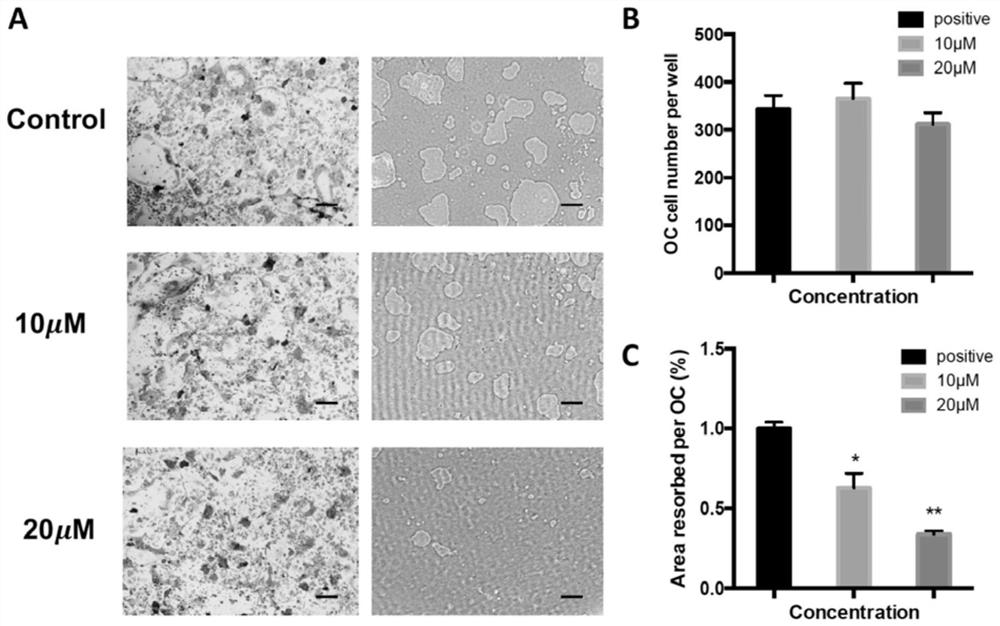
Application of asiaticoside in the treatment of abnormal bone metabolism
ActiveCN108159060BInhibition formationDysregulation of bone metabolismOrganic active ingredientsSkeletal disorderPharmacological interventionsPharmacometrics
The invention discloses asiaticoside, and a function of a pharmaceutically acceptable derivative thereof in bone metabolic abnormality. The asiaticoside has an adjustment function on simultaneously inhibiting osteoclast formation under the situation of bone metabolic abnormality, is low in effective dosage, and has a better effect on adjusting bone metabolic abnormality. The invention defines an intervention way of an asiaticoside related drug. The invention clarifies that a molecular mechanism in a bone microenvironment and a potential pharmacology intervention way are crucial to identifyinga novel treatment compound.
Owner:THE FIRST AFFILIATED HOSPITAL OF GUANGZHOU UNIV OF CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com