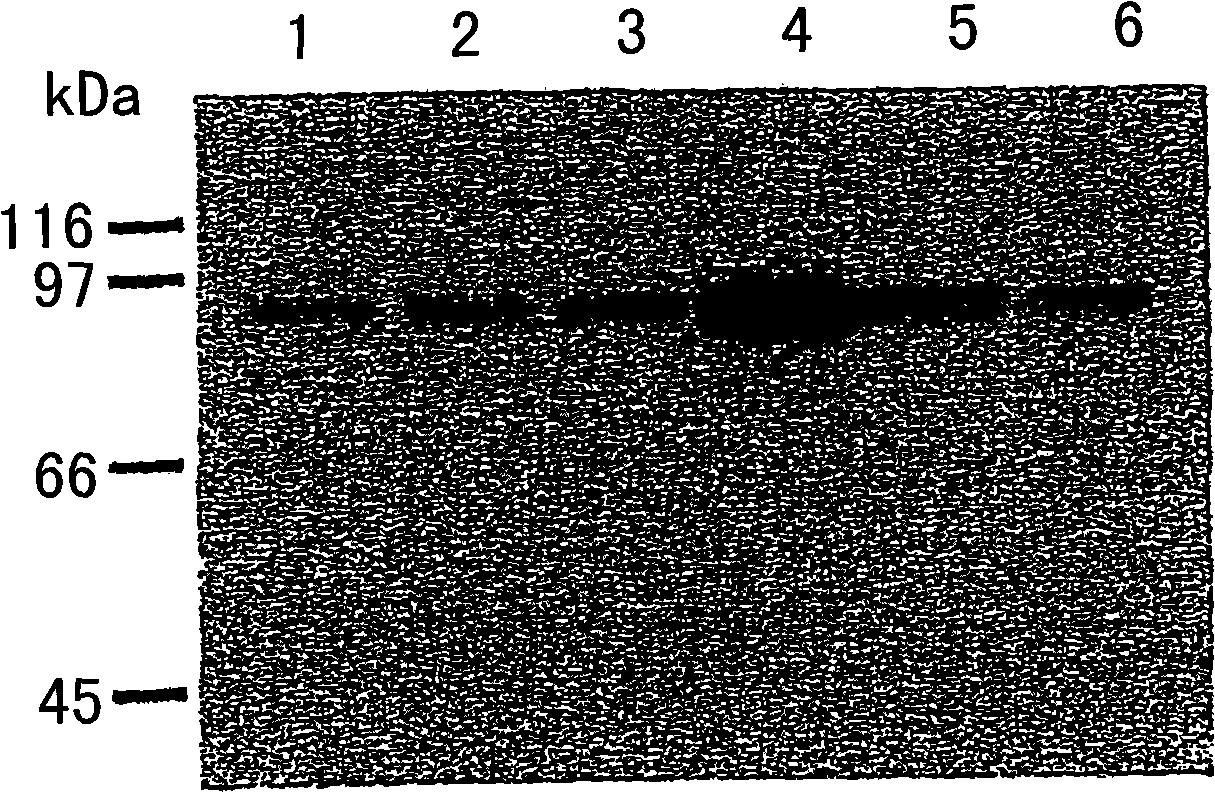
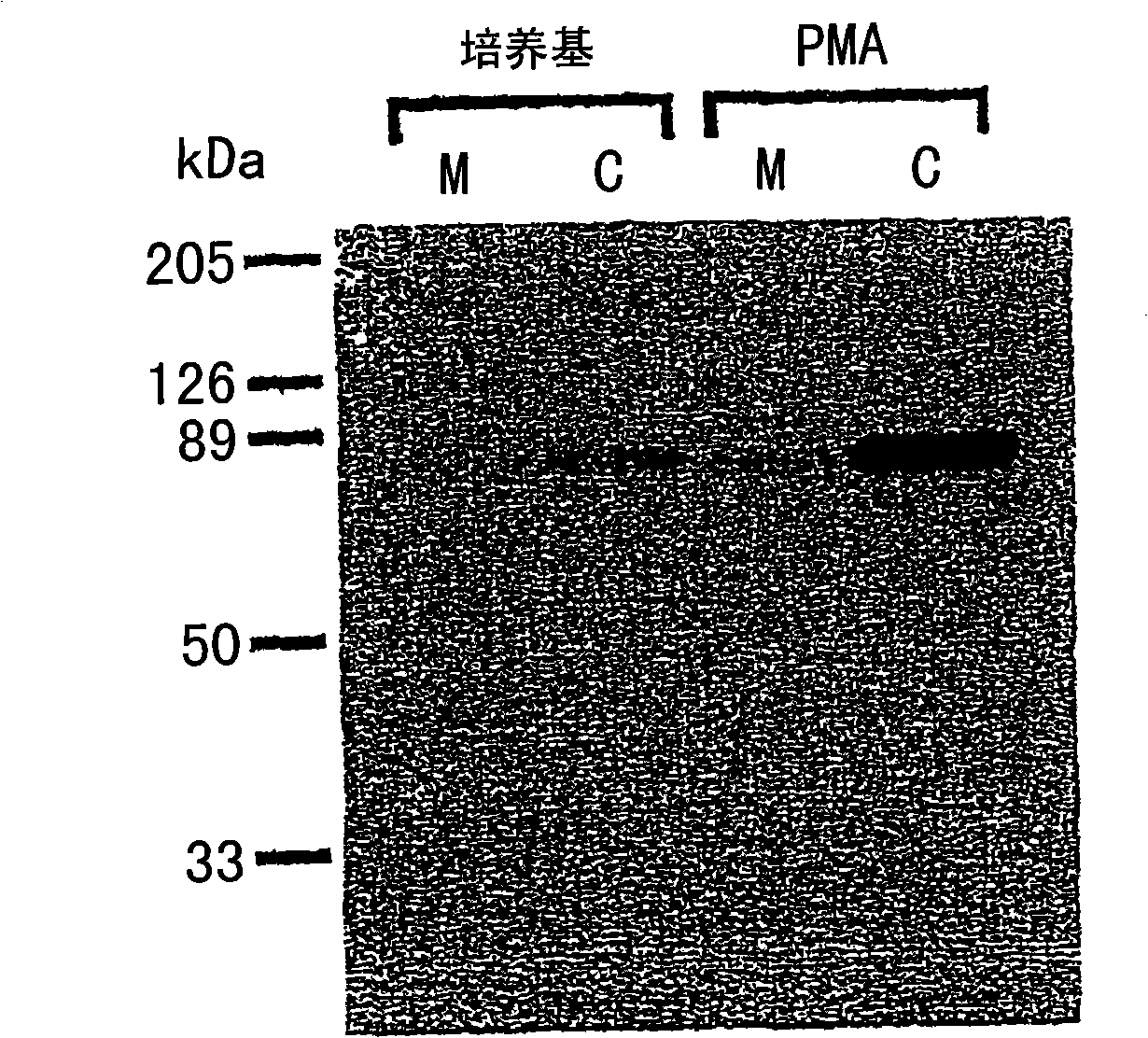
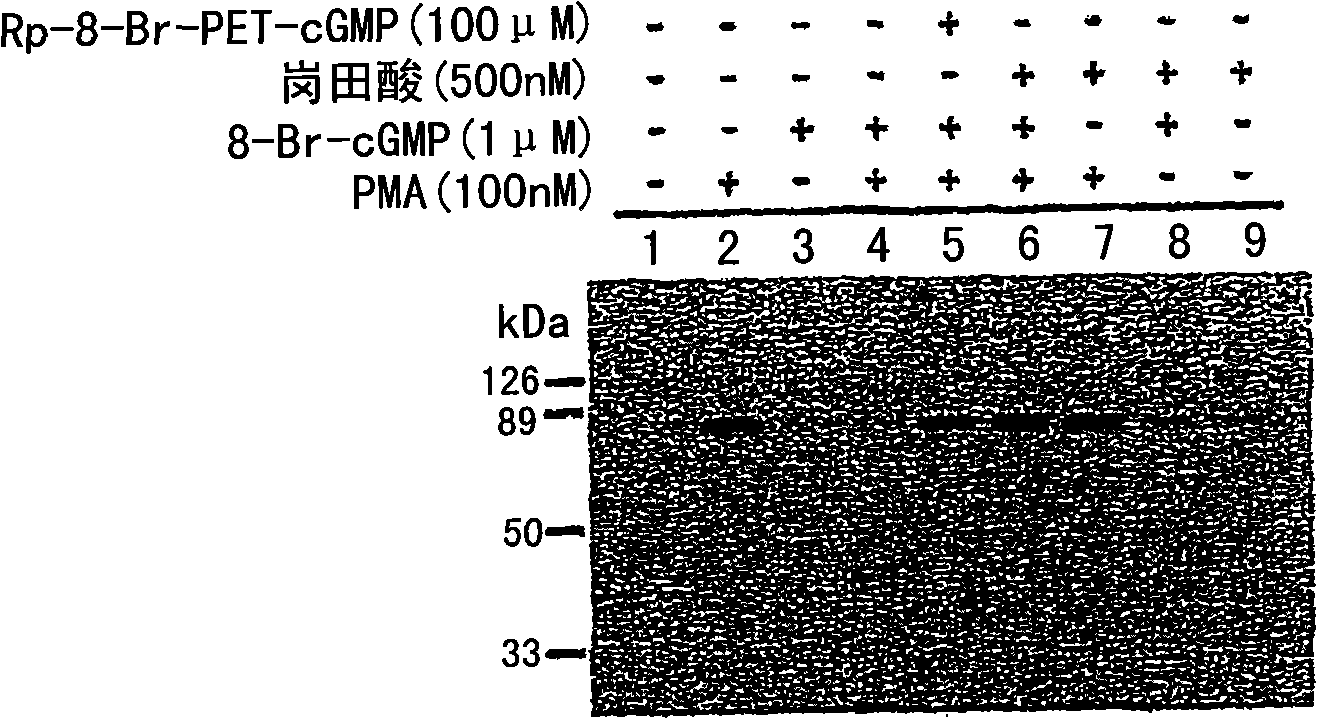
Methods for attenuating release of inflammatory mediators and peptides useful therein
A technology of mediators and inflammation, applied in the field of modulation and regulation of intracellular signaling mechanisms that regulate the secretion of inflammatory mediators from inflammatory cells, can solve the problems of insufficiently revealing regulatory molecules and specific pathways, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0452] Example 1: Study on the secretion of inflammatory mediators
[0453] Four different white blood cell types or models that secrete specific granular components in response to phorbol ester-induced PKC activation were used. Neutrophils were isolated from human blood and tested for the release of MPO from these cells in vitro. The release of membrane-bound inflammatory mediators from a commercial human leukocyte cell line was also evaluated. The human promyelocytic cell line HL-60 clone 15 was used to analyze the release of EPO (Fischkoff SA. Graded increase in probability of eosinophilic differentiation of HL-60 promyelocyticleukemia cells induced by culture under alkaline conditions. Leuk Res 1988; 12: 679-686; Rosenberg HF , Ackerman SJ, Tenen DG. Human eosinophilcationic protein: molecular cloning of a cytotoxin and helminthotoxin withribonuclease activity. J Exp Med 1989; 170: 163-176; Tiffany HL, Li F, Rosenberg HF. Hyperglycosylation of eosinophil ribonucleases in a cel...
Embodiment 2
[0490] Example 2: MANS and related peptides inhibit lipopolysaccharide (LPS)-induced lung inflammation in vivo
[0491] This example is basically carried out according to the method described in the following documents: Cox, G, Crossley, J., and Xing, Z.; Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in ViVo; Am.J.Respir.Cell Mol. Biol. 12: 232-237, 1995; Hirano S., Quantitative time-course profiles of bronchoalveolar laVage cells following intratracheal instillation of lipopolysaccharide in mice, Ind. Health 35: 353-358, 1997; and Ulich TR, WatsonLR, Yin SM , Guo KZ, Wang P, Thang H, and del Castillo, J. Am. J. Pathol. 138: 1485-1496, 1991.
[0492] Therefore, 6 to 7-week-old CD1 female mice weighing 15-20 g were obtained from Charles River Laboratories, and 5 mice per cage were raised in groups. The animals received ad libitum standard rodent diet and filtered water. Follow NIH guidelines to keep animals at standard ...
Embodiment 3
[0507] Example 3: Ozone-induced COPD mouse model
[0508] Oxidative stress caused by chemical irritants such as ozone is a widely recognized feature of chronic obstructive respiratory disease (COPD). See: Repine JE, Bast A, Lankhorst I, and the Oxidative Stress Study Group, Am.J.Respir.Crit.Care Med.156:341-357, 1997; and also Harkema JR and Hotchkiss JA, Toxicology Letters, 68:251- 263, 1993.
[0509] 10-week-old Balb / C female mice were obtained from Charles River Laboratories, and 5 mice per cage were raised in groups according to NIH guidelines. The animals received ad libitum standard rodent diet and filtered water. Mice in 3 treatment groups, 5 in each group, were anesthetized by intraperitoneal injection of ketamine (100mg / kg) and xylazine (20mg / kg), and then pretreated by intratracheal administration of 25 μL of the following treatment: PBS only; Or 1.0 mM MANS peptide solution dissolved in PBS; or 1.0 mM acetylated MANS fragment peptide Ac-GAQFSKTAAK (named acetylated SEQ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com