Chlorpheniramine maleate compound and pharmaceutical composition thereof
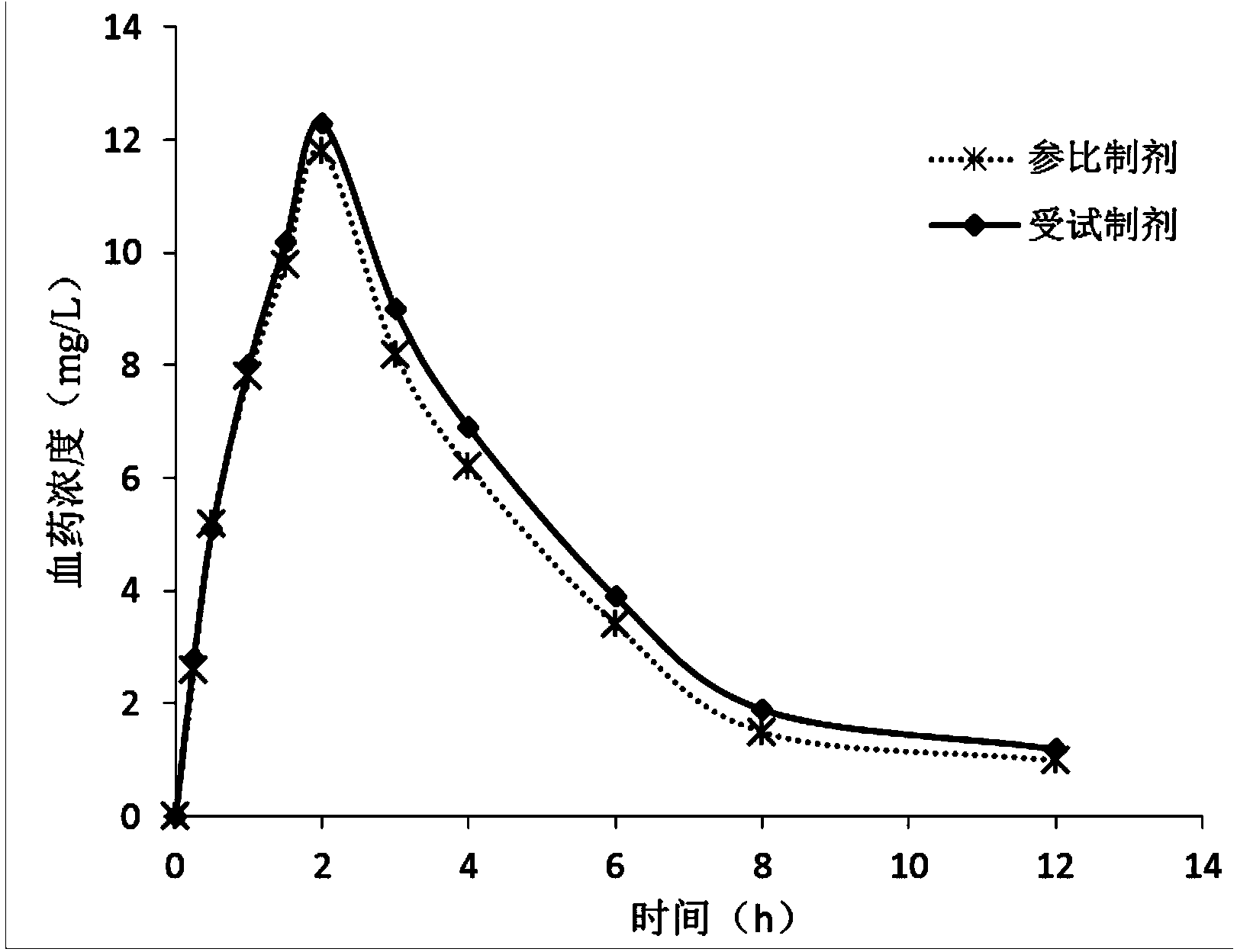
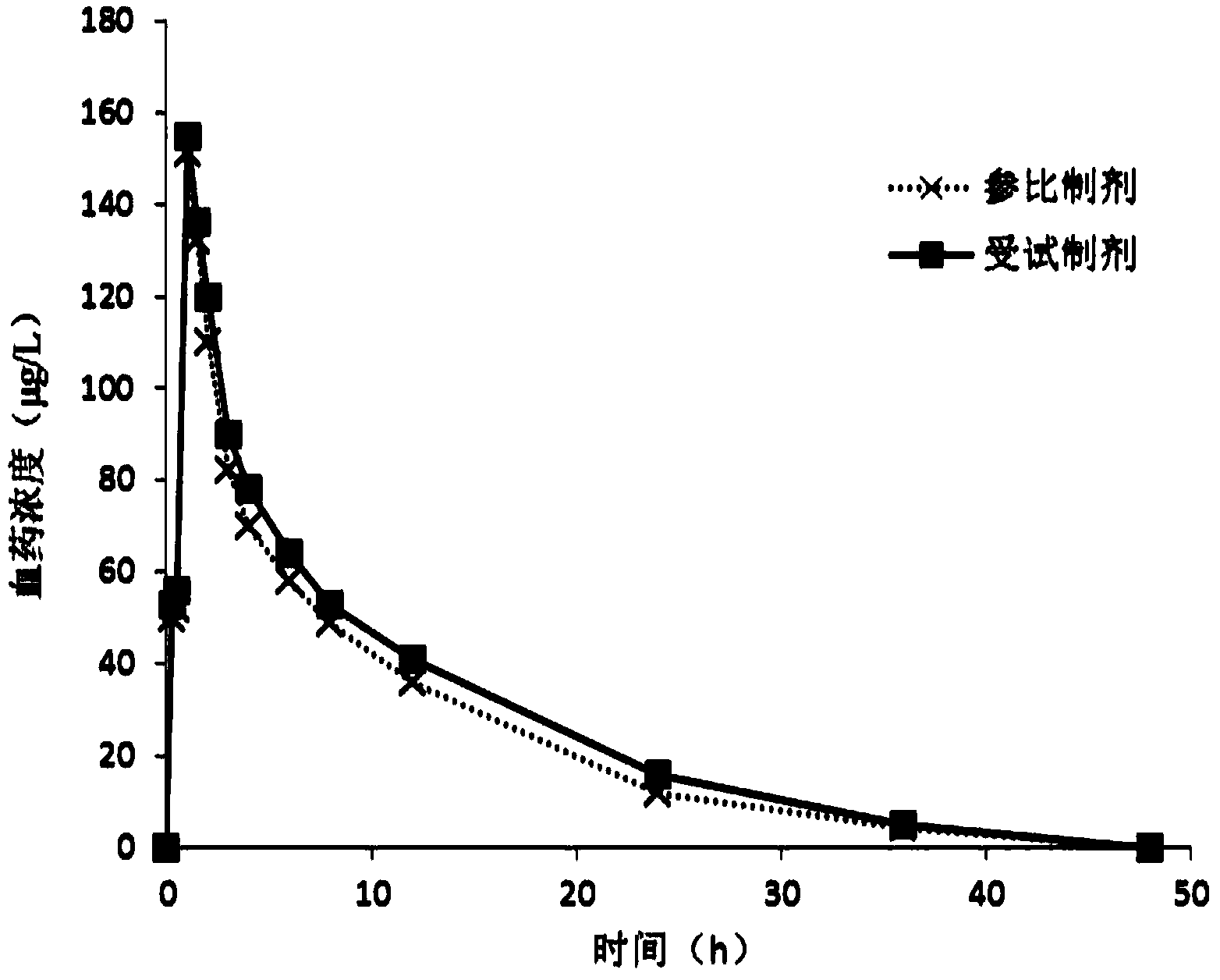
A technology of chlorpheniramine acid compound and composition, which is applied in the field of chlorpheniramine maleate compound and pharmaceutical composition thereof, can solve problems such as complicated preparation method, and achieves enhancement of drug efficacy, narrowing of gap and peak time and half-life shortening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] [embodiment 1] the preparation of chlorpheniramine maleate compound
[0056] 1) Prepare the crude product solution: add the crude chlorpheniramine maleate into a mixed solvent prepared from chloroform and ethanol, heat to 50°C, stir to dissolve, filter to remove insoluble particles, and obtain a crude product solution for later use;
[0057] 2) Preparation of a crystallization solvent: prepare a crystallization solvent with acetone and diethyl ether at a volume ratio of 1.0:10, and the volume of the crystallization solvent is 10 times the weight of the crude chlorpheniramine maleate;
[0058] 3) Crystallization: under stirring, add the crystallization solvent obtained in step 2) to the crude product solution obtained in step 1), and solids precipitate out; 4 times the weight of the crude product of pheniramine, the temperature of the solution is controlled at 50°C during the whole dropping process; after dropping, cool to 5°C and let stand for 3h, filter, wash, and dry ...
Embodiment 2-9
[0062]
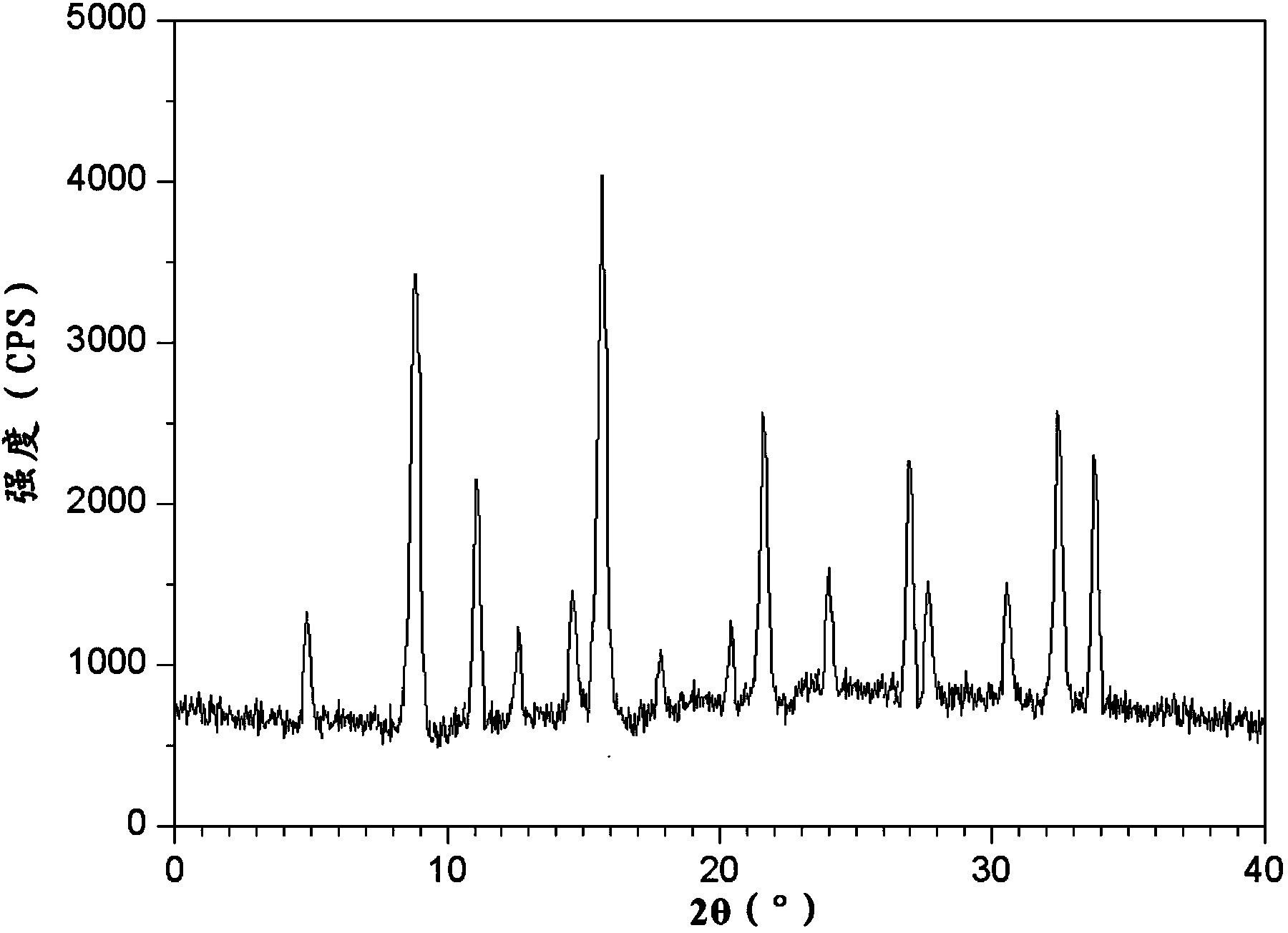
[0063] The obtained chlorpheniramine maleate compound of embodiment 2-9 is measured with powder X-ray diffractometry, and the X-ray powder diffraction pattern that represents with 2θ ± 0.2 ° diffraction angle is the same as embodiment 1.
preparation Embodiment 1
[0064] [Preparation Example 1] Preparation of Ibuprofen Pseudopheniramine Tablets
[0065] prescription:
[0066] Tablets:
[0067]
[0068] Preparation:
[0069] (1) Weigh the raw and auxiliary materials according to the stated dosage; pulverize the ibuprofen and pseudoephedrine hydrochloride so that they all pass through a 80-mesh sieve, and set aside; grind the chlorpheniramine maleate through a 100-mesh sieve, and set aside;
[0070] (2) Preparation of adhesive: take another 12.1g of starch, add 18.15g of purified water, stir and mix to obtain a starch suspension; add 1.8g of polysorbate 80 to 102.85g of purified water and boil it, then add it under stirring Put it into the starch suspension to get starch paste, weigh it, subtract the actual weight from the theoretical weight of 134.9g, calculate the purified water lost in the process of boiling water, supplement the lost part to the theoretical amount, and make 10% starch paste, put Cool to 50-60°C for later use;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com