Extended release tablet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
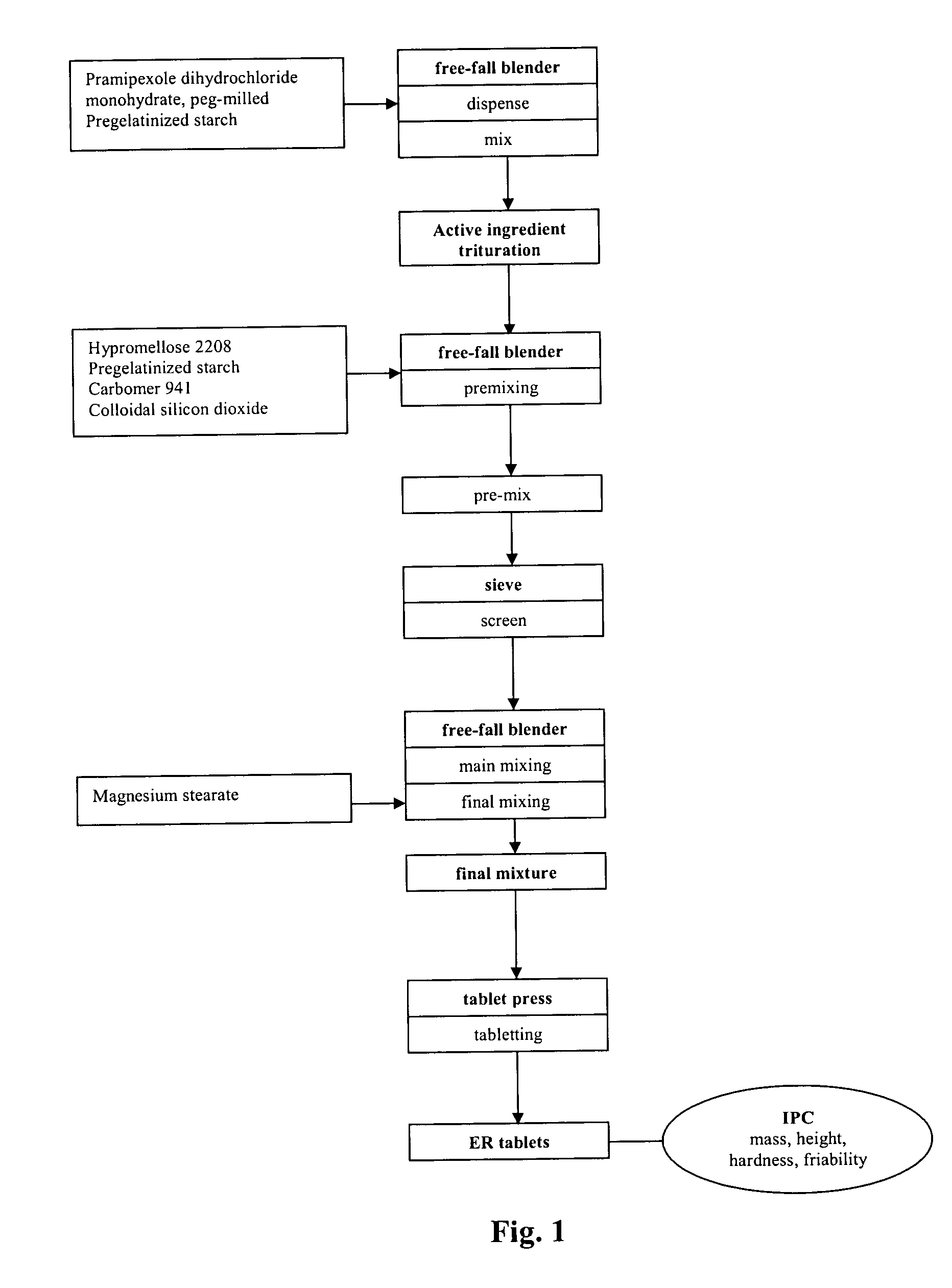
[0107] One embodiment of the qualitative and quantitative composition of pramipexole extended release tablets according to the present invention is shown in Table 1.
TABLE 1Qualitative and Quantitative Composition ofPramipexole Extended Release Tabletmg per 0.75Reference toIngredientmg tabletFunctionStandardsPramipexole dihydrochloride0.750ActiveCorporatemonohydrate, peg-milledingredientstandardHypromellose 2208157.500SwellingPh.Eur. / USP(Methocel K 15 M)agentPregelatinized starch185.100FillerPh.Eur. / NF(Starch 1500)Carbomer 9413.500GellingPh.Eur. / NF(CARBOPOL ® 71 G)agentColloidal silicon dioxide1.400GlidantPh.Eur. / NFMagnesium stearate1.750LubricantPh.Eur. / NFTotal350.000
example 2
[0108] A further embodiment of the qualitative and quantitative composition of pramipexole extended release tablets according to the present invention is shown in Table 2.
TABLE 2Qualitative and Quantitative Compositionof Pramipexole Extended Release Tabletmg per 0.75Reference toIngredientmg tabletFunctionStandardsPramipexole dihydrochloride0.750ActiveCorporatemonohydrate, peg-milledingredientstandardHypromellose 2208157.500SwellingPh.Eur. / USP(Methocel K 15 M)agentPregelatinized starch174.600FillerPh.Eur. / NF(Starch 1500)Carbomer 94114.000GellingPh.Eur. / NF(CARBOPOL ® 71 G)agentColloidal silicon dioxide1.400GlidantPh.Eur. / NFMagnesium stearate1.750LubricantPh.Eur. / NFTotal350.000
example 3
[0109] The batch formula for the two pramipexole tablet formulations of Example 1 and 2 is shown in Table 3. The batch size of the final mixture corresponds to a batch size of 2000 tablets.
TABLE 3Composition per Batch of Pramipexole 0.75 mg ER TabletsGrams perGrams perbatchbatchIngredientExample 1Example 2Pramipexole dihydrochloride1.5001.500monohydrate, peg-milledHypromellose 2208315.000315.000Pregelatinized starch370.200349.200Carbomer 9417.00028.000Colloidal silicon dioxide2.8002.800Magnesium stearate3.5003.500Total Mass700.000700.000
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com