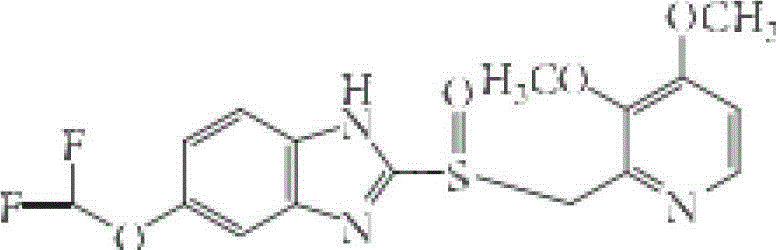
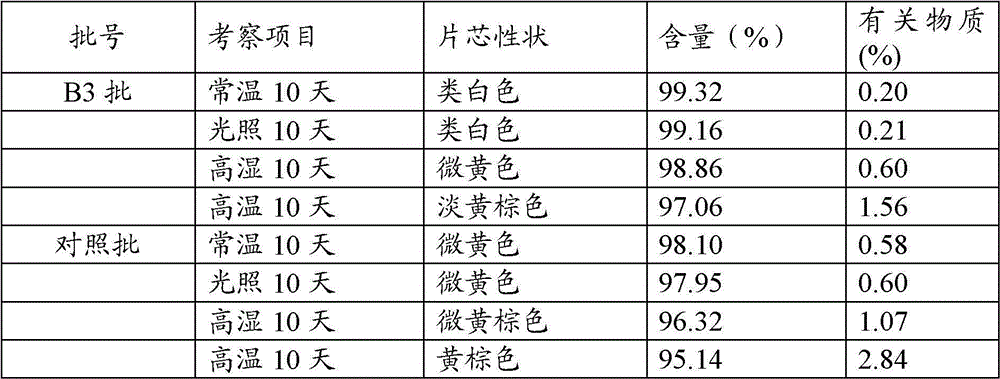
Enteric-coated tablet of S-pantoprazole or salt of S-pantoprazole, and preparation method thereof
A technology of pantoprazole salt and pantoprazole, which is applied in the field of pharmaceutical dosage forms, can solve the problems of infusion drug efficacy decline, capillary embolism, and poor firmness, and achieve the effects of accurate dosage, high bioavailability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Prescription (make 5000 tablets):
[0096] (1) Chip core:
[0097]
[0098] (2) Isolation layer (1000g):
[0099] Opadry (03K19229) 50g
[0100] 80% ethanol 350g
[0101] (3) Enteric-coated layer (1000g):
[0102] Opadry (94O62488) 100g
[0103] 85% ethanol 900g
[0104] Preparation:
[0105] 1) Preparation of tablet core
[0106] Get the S-pantoprazole sodium two times hemihydrate of recipe quantity, microcrystalline cellulose, lactose, croscarmellose sodium, anhydrous sodium carbonate, cross 65 mesh sieves and mix homogeneously; According to the actual prescription powder obtained amount, add an appropriate amount of magnesium stearate and talcum powder, mix uniformly, and the powder is directly compressed into tablets to obtain the tablet core of S-pantoprazole sodium.
[0107] 2) Preparation of isolation layer coating solution
[0108] Prepare ethanol with a concentration of 80%, dissolve Opadry (03K19229) in the ethanol solution, and mix well to obtain ...
Embodiment 2
[0114]Prescription (make 5000 tablets):
[0115] (1) Chip core:
[0116]
[0117] (2) Isolation layer (1000g):
[0118] Opadry (17K690000) 50g
[0119] 85% ethanol 350g
[0120] (3) Enteric-coated layer (1000g):
[0121] Opadry (94O62488) 100g
[0122] 85% ethanol 900g
[0123] Preparation:
[0124] 1) Preparation of tablet core
[0125] Get the prescription amount of S-pantoprazole magnesium, microcrystalline cellulose, lactose, croscarmellose sodium, anhydrous sodium carbonate, pass through a 65 mesh sieve and mix evenly; Magnesium fatty acid and talcum powder are uniformly mixed, and the powder is directly compressed into tablets to obtain the tablet core of S-pantoprazole magnesium.
[0126] 2) Preparation of isolation layer coating solution
[0127] Prepare ethanol with a concentration of 85%, dissolve Opadry (17K690000) in the ethanol solution, and mix well to obtain a coating solution for the isolation layer.
[0128] 3) Preparation of coating liquid
[01...
Embodiment 3
[0133] Prescription (make 5000 tablets):
[0134] (1) Chip core:
[0135]
[0136] (2) Isolation layer (1000g):
[0137] Opadry (17K690000) 50g
[0138] 75% ethanol 350g
[0139] (3) Enteric-coated layer (1000g):
[0140] Opadry (94O62488) 100g
[0141] 80% Alcohol 900g
[0142] Preparation:
[0143] 1) Preparation of tablet core
[0144] Get the S-pantoprazole magnesium dihydrate, microcrystalline cellulose, lactose, croscarmellose sodium, anhydrous sodium carbonate of prescription quantity, cross 65 mesh sieves and mix; According to the actual prescription powder quantity of gained, Add appropriate amount of magnesium stearate and talcum powder, mix evenly, and directly compress the powder into tablets to obtain the tablet core of S-pantoprazole magnesium.
[0145] 2) Preparation of isolation layer coating solution
[0146] Prepare ethanol with a concentration of 75%, dissolve Opadry (17K690000) in the ethanol solution, and mix well to obtain a coating solution f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com