Montelukast sodium chewing tablet prescription and preparation process thereof
A technology of montelukast sodium and chewable tablets, which is applied in the field of symptomatic drugs, montelukast sodium chewable tablet formulations, which can solve the problems of difficulty in adapting to children, long drug residence time, and slow melting speed in the mouth. Good fluidity and compressibility, shortened production process cycle, and improved patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
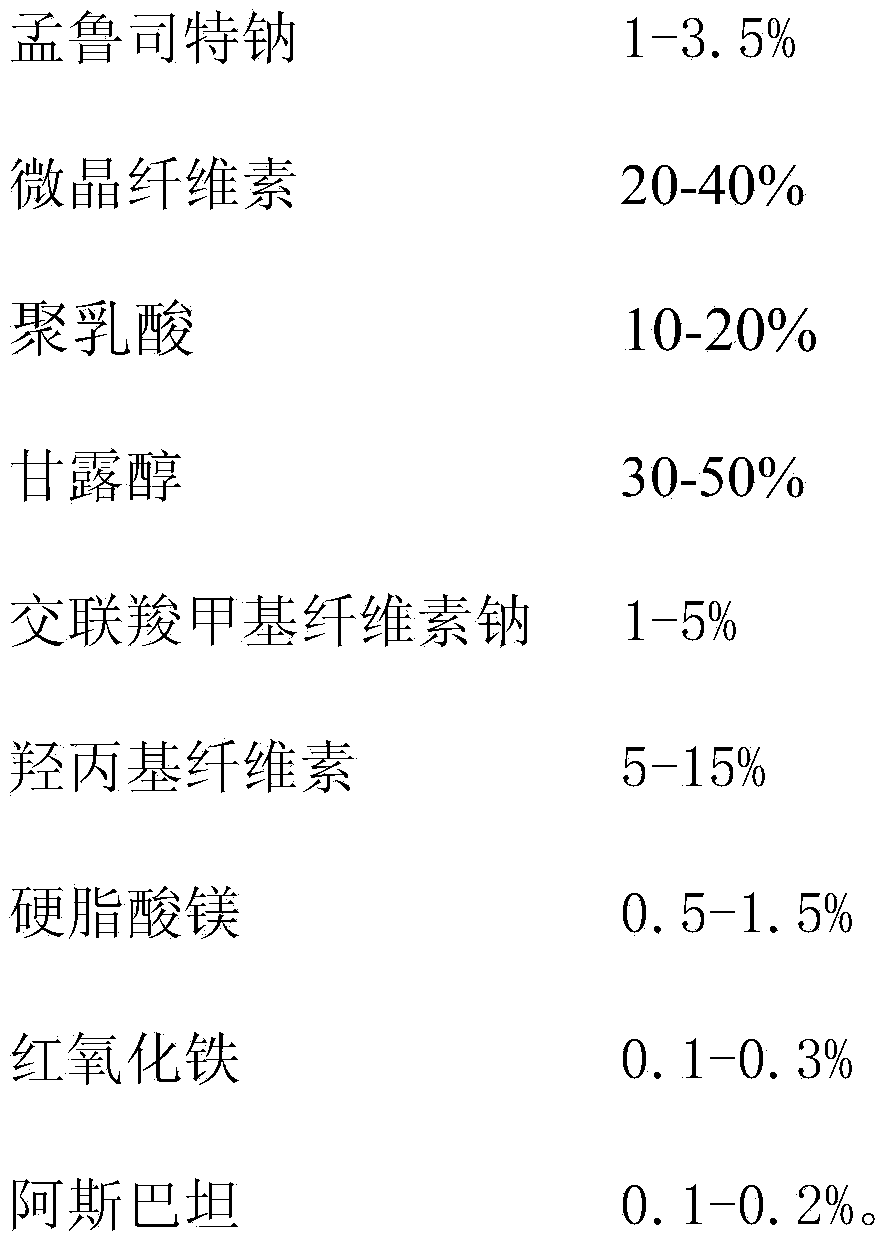
[0051]
[0052]
[0053] Preparation:
[0054] 1) Montelukast sodium API, microcrystalline cellulose, polylactic acid, mannitol, croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, red iron oxide, aspartame Pass through 80 mesh sieve respectively.
[0055] 2) Take montelukast sodium, microcrystalline cellulose, polylactic acid, mannitol, 3 g of croscarmellose sodium, hydroxypropyl cellulose, red iron oxide, and aspartame in equal increments After being put into a three-dimensional motion mixer and mixed for 50 minutes, magnesium stearate was added and mixed for 5 minutes. The mixed material is put into the hopper of a high-speed rotary tablet press, and the tablet is pressed with an appropriate tablet weight and pressure.
Embodiment 2
[0057]
[0058] 1) Montelukast sodium API, microcrystalline cellulose, polylactic acid, mannitol, croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, red iron oxide, aspartame Pass through 80 mesh sieve respectively.
[0059] 2) Take montelukast sodium, microcrystalline cellulose, polylactic acid, mannitol, 4.8 g of croscarmellose sodium, hydroxypropyl cellulose, red iron oxide, and aspartame in equal increments Method Put it into a three-dimensional motion mixer and mix for 50 minutes, then add magnesium stearate and mix for 5 minutes. The mixed material is put into the hopper of a high-speed rotary tablet press, and the tablet is pressed with an appropriate tablet weight and pressure.
Embodiment 3
[0061]
[0062] Preparation:
[0063] 1) Montelukast sodium API, microcrystalline cellulose, polylactic acid, mannitol, croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, red iron oxide, aspartame Pass through 80 mesh sieve respectively.
[0064] 2) Take montelukast sodium, microcrystalline cellulose, polylactic acid, mannitol, croscarmellose sodium, hydroxypropyl cellulose, red iron oxide, and aspartame in equal increments, After being put into a three-dimensional motion mixer and mixed for 50 minutes, magnesium stearate was added and mixed for 5 minutes. The mixed material is put into the hopper of a high-speed rotary tablet press, and the tablet is pressed with an appropriate tablet weight and pressure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com