Tofacitinib citrate tablets and preparation method thereof
A technology of tofacitinib and citric acid, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor stability, uneven product content, and slow dissolution rate, etc. problems, to achieve the effect of fast dissolution rate, high content uniformity and stable chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
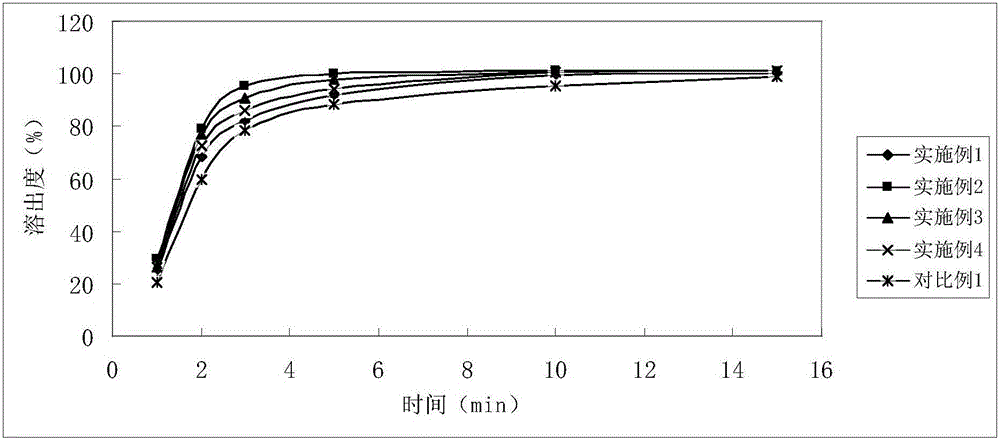
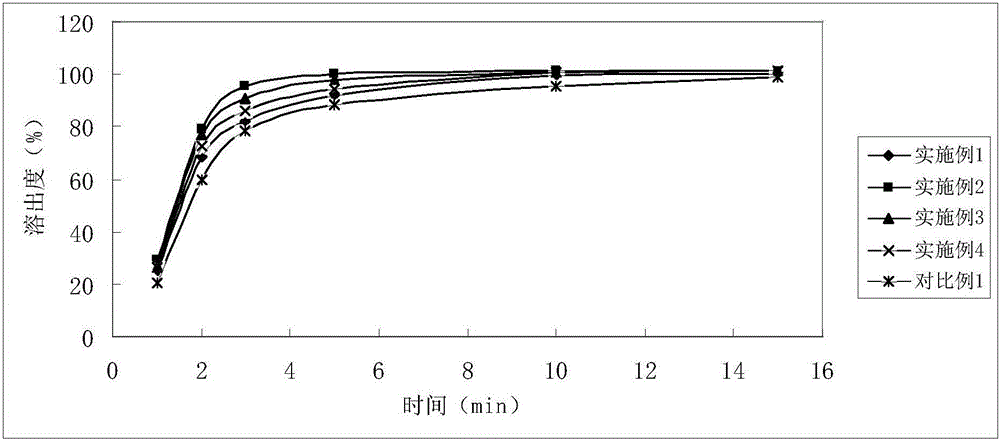
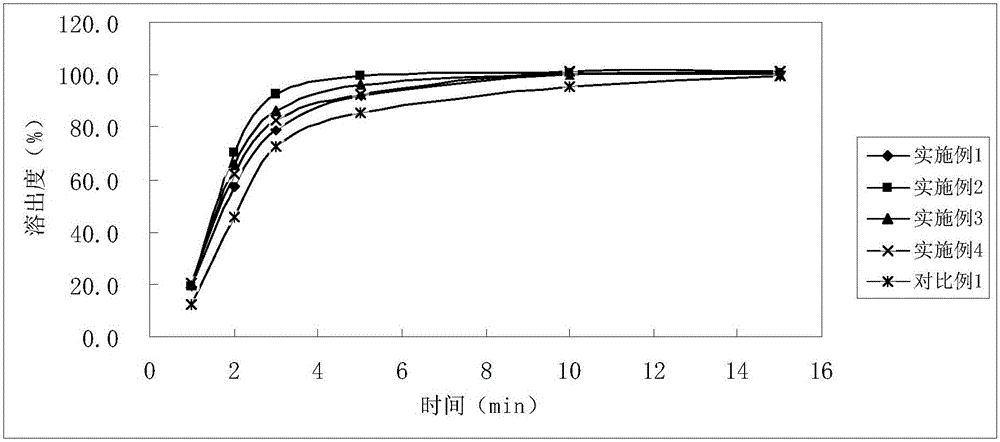
Examples
Embodiment 1
[0025] The prescription of table 1 embodiment 1
[0026] type
name
%(w / w)
API
13.0
filler
Celactose 80
78.1
disintegrant
4.9
silica
1.0
Coating agent
Gastric film coating premix
3.0
[0027] The preparation method is as follows:
[0028] 1) Weigh the raw material and auxiliary materials in proportion, and crush tofacitinib citrate to a particle size of 10-30 μm;
[0029] 2) Mix the tofacitinib citrate powder with the microcrystalline cellulose lactose premix whose model is Celactose80 first, then add croscarmellose sodium and mix evenly; finally add silicon dioxide and mix evenly;
[0030] 3) Tablet pressing: when pressing, the ambient temperature is controlled to be 15°C, the humidity is 60%, and the pressure of the tablet press is 25KN;
[0031] 4) Dissolving the stomach-soluble film coating premix with 60% ethanol, and then applyi...
Embodiment 2
[0033] The prescription of table 2 embodiment 2
[0034] type
name
%(w / w)
API
Tofacitinib citrate
8.5
filler
Celactose 80
81.3
disintegrant
Low-substituted hydroxypropyl cellulose
4.3
1.9
Coating agent
Gastric film coating premix
4
[0035] The preparation method is as follows:
[0036] 1) Weigh the raw material and auxiliary materials in proportion, and crush tofacitinib citrate to a particle size of 40-60 μm;
[0037] 2) Mix the tofacitinib citrate powder with the microcrystalline cellulose lactose premix whose model is Celactose80, then add low-substituted hydroxypropyl cellulose and mix evenly; finally add magnesium stearate and mix evenly;
[0038] 3) Tablet compression: when the tablet is pressed, the ambient temperature is controlled to be 20°C, the humidity is 40%, and the pressure of the tablet press is 30KN;
Embodiment 3
[0041] The prescription of table 3 embodiment 3
[0042] type
name
%(w / w)
API
Tofacitinib citrate
4.4
filler
Celactose 80
87.2
disintegrant
Cross-linked polyvinylpyrrolidone
2.8
talcum powder
1.1
Coating agent
Gastric film coating premix
4.5
[0043] The preparation method is as follows:
[0044] 1) Weigh the raw materials and auxiliary materials in proportion, and crush tofacitinib citrate to a particle size of 70-90 μm;
[0045] 2) Mix tofacitinib citrate powder with the microcrystalline cellulose lactose premix whose model is Celactose80 first, then add cross-linked polyvinylpyrrolidone and mix evenly; finally add talc powder and mix evenly;
[0046] 3) Tablet compression: control the ambient temperature to be 10° C., the humidity to be 50% during tablet compression, and the pressure of the tablet press to be 28KN;
[0047] 4) Dissolving the stomach-soluble film-coating p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com