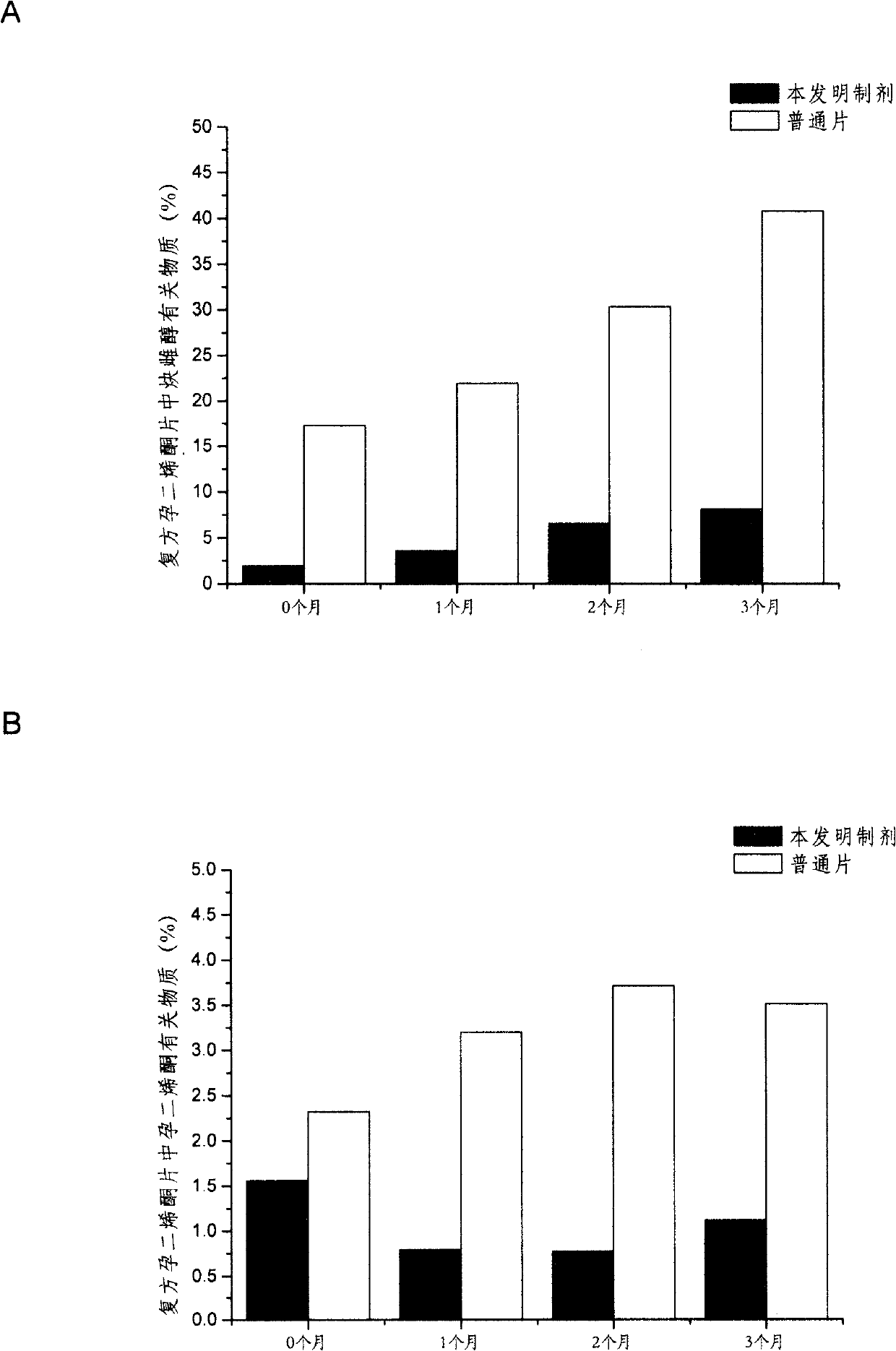
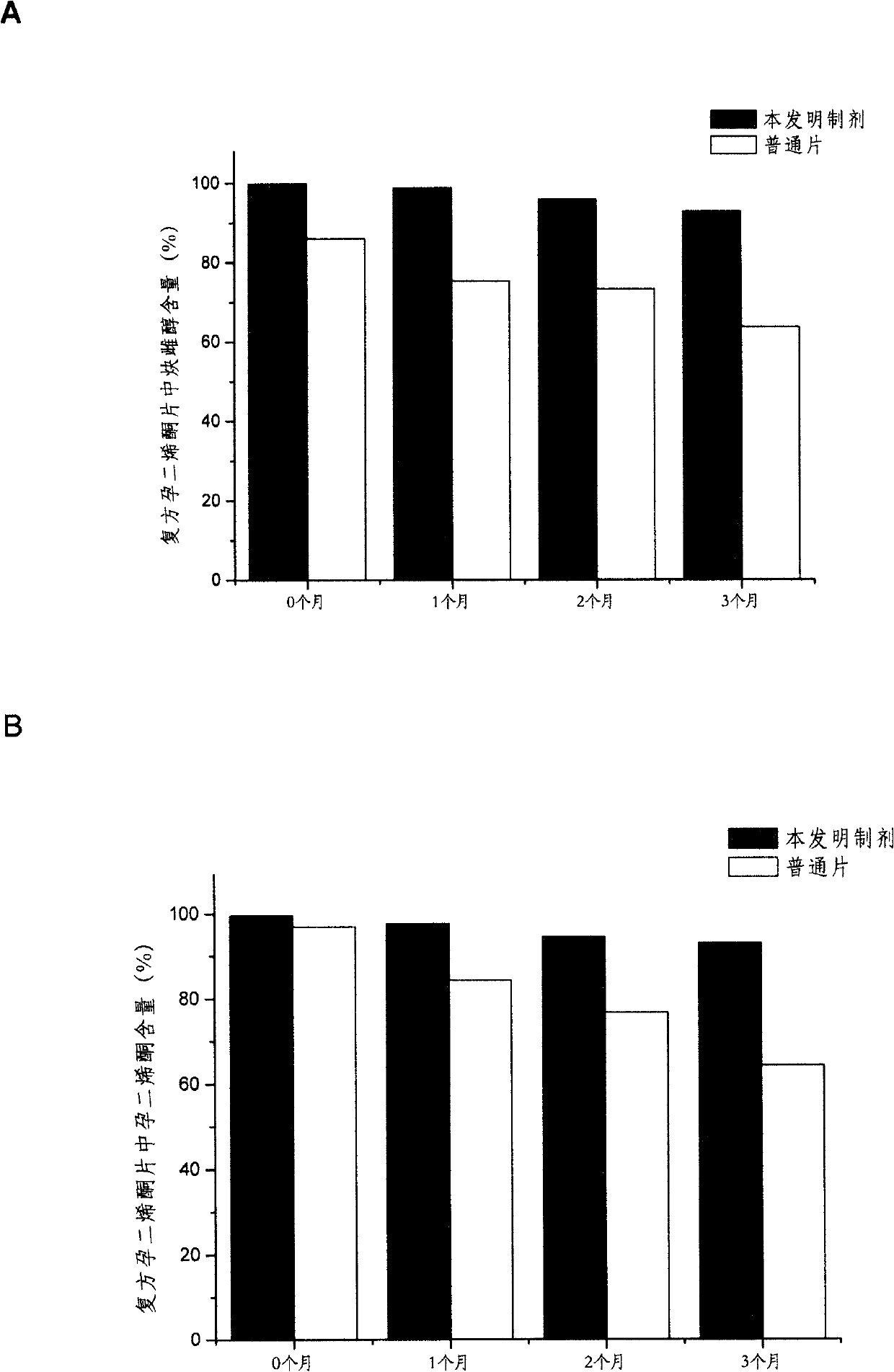
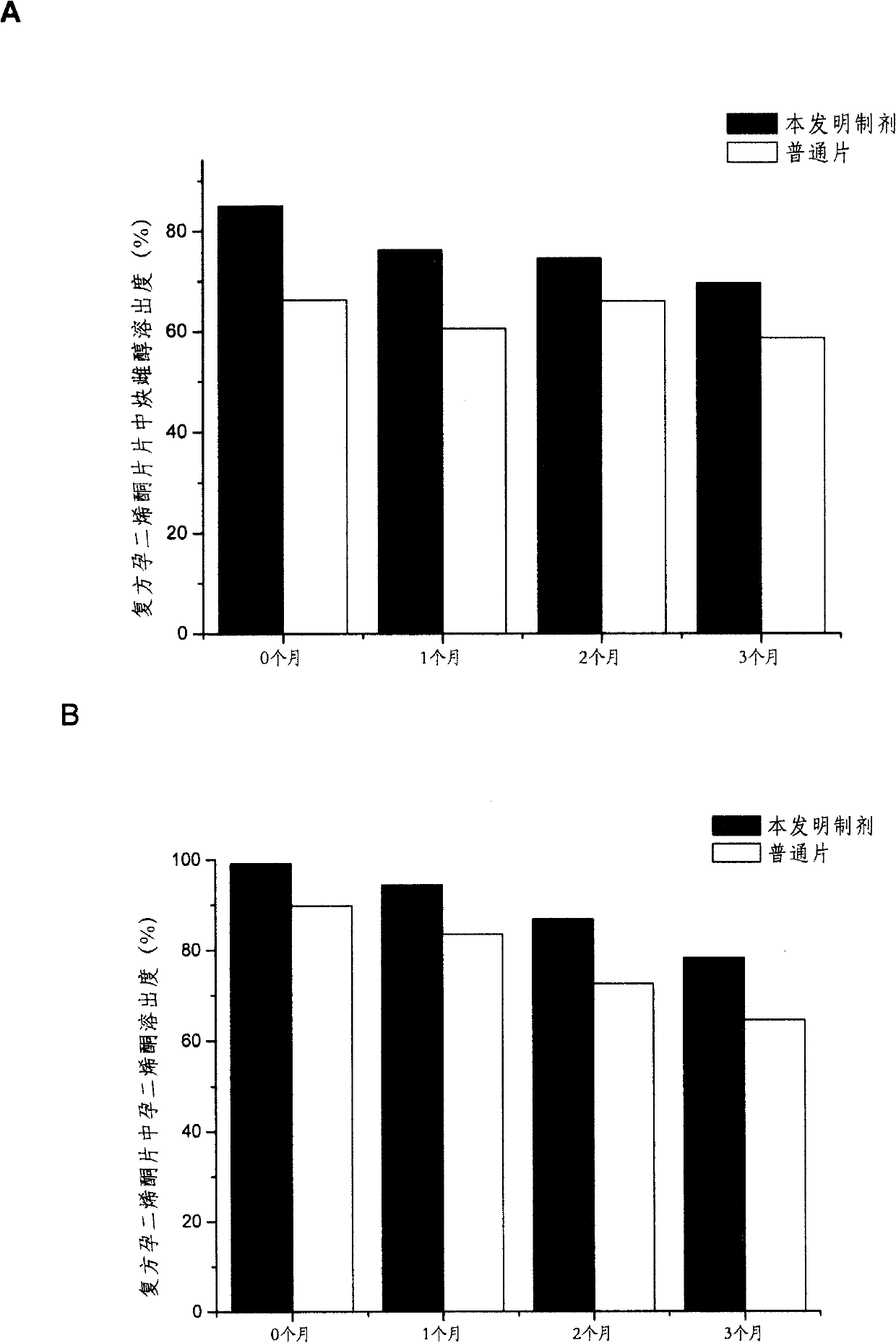
Solid dispersoid containing progestational hormone, preparation method thereof and composite comprising same
A solid dispersion, progesterone technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Take 1000 pieces as an example:
[0034] Gestodene (Beijing Zizhu Pharmaceutical Co., Ltd.) 75mg
[0035] PVP (PLASDON K-29 / 32, American ISP Company) 3.75g
[0036] Ethinylestradiol (Shanghai Pharmaceutical Group Co., Ltd.) 30mg
[0037] Calcium Carbonate (Shanghai Daewoo Biochemical Co., Ltd.) 63g
[0038] Cross-linked PVP (Polyplasdone XL or XL-10, American ISP company, 12g
[0039] Mw=10,000-300,000)
[0040] Magnesium stearate [China Pharmaceutical (Group) Shanghai Chemical Reagent Company] 1g
[0041] Dissolve Gestodene and PVP in 50 mL of ethanol at the same time, or dissolve them in ethanol separately and mix well. Remove the solvent by vacuum drying to obtain about 3.75 g of solid dispersion.
[0042] Pulverize the above solid dispersion, pass through a 60-mesh sieve, mix evenly with ethinyl estradiol and calcium carbonate, granulate with 75% ethanol aqueous solution, dry (45°C), add cross-linked PVP and magnesium stearate for granulation, and tablet. Get ...
Embodiment 2
[0044] Take 1000 pieces as an example:
[0045] Gestodene (Beijing Zizhu Pharmaceutical Co., Ltd.) 60mg
[0046] Ethinylestradiol (Shanghai Pharmaceutical Group Co., Ltd.) 20mg
[0047] PVP (PLASDON K-29 / 32, American ISP Company) 3g
[0048] Low-substituted hydroxypropyl methylcellulose (Huzhou Zhanwang Tianming Pharmaceutical Co., Ltd.) 12g
[0049] Compressible starch (Starch 1500, American Colorcon company) 63g
[0050] Talc powder (Dandong Medicinal Talc Powder Factory) 0.5g
[0051] Magnesium stearate (Anhui Shanhe Pharmaceutical Excipients Co., Ltd.) 0.5g
[0052] Dissolve Gestodene and PVP in 40 mL of ethanol at the same time, or dissolve in ethanol separately and mix well, and remove the solvent by vacuum drying (vacuum condition is less than 20 mmHg) to obtain about 3 grams of solid dispersion.
[0053] Crush the above solid dispersion, pass through a 60-mesh sieve, mix evenly with ethinyl estradiol, low-substituted hydroxypropylmethylcellulose (L-HPC) and compress...
Embodiment 3
[0055] Take 1000 pieces as an example:
[0056] Gestodene (Beijing Zizhu Pharmaceutical Co., Ltd.) 60mg
[0057] PVP (K30, Hainan Nanhang Pharmaceutical Co., Ltd.) 6g
[0058] Ethinylestradiol (Shanghai Xinyi Pharmaceutical Co., Ltd.) 20mg
[0059] Calcium carbonate (Shandong Liaocheng Anxin Pharmaceutical Excipients Co., Ltd.) 60g
[0060] Cross-linked PVP (Hainan Nanhang Pharmaceutical Co., Ltd.) 12g
[0061] Magnesium stearate (Shandong Liaocheng Ahua Pharmaceutical Co., Ltd.) 1g
[0062] Dissolve Gestodene and PVP in 50 mL of ethanol at the same time, or dissolve them in ethanol separately and mix well. Remove the solvent by vacuum drying (vacuum degree less than 20 mmHg) to obtain about 6 grams of solid dispersion.
[0063] Pulverize the above solid dispersion, pass through a 60-mesh sieve, mix evenly with ethinyl estradiol and calcium carbonate, granulate with 75% ethanol aqueous solution, dry (45°C), add cross-linked PVP and magnesium stearate for granulation, and t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com