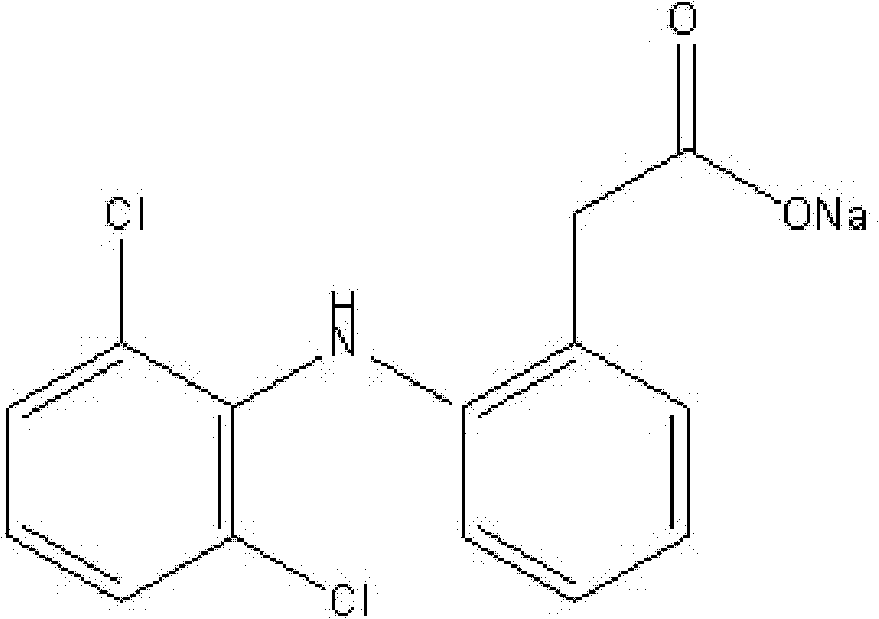
Diclofenac sodium sustained-release tablet and its preparation process
A technology for diclofenac sodium and its preparation process, which is applied in the field of diclofenac sodium sustained-release tablets and its preparation, and can solve problems such as limitations in the industrial production and application of the full powder direct compression method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] formula:
[0076]
[0077] Preparation Process:
[0078] Accurately weigh 1000.0g of diclofenac sodium, 567.5g of hydroxypropyl methylcellulose, 403.0g of methylcellulose, 1341.1g of calcium hydrogen phosphate, 152.0g of micropowder silica gel, 50.0g of talcum powder, 70.6g of magnesium stearate, Ouba Substitute (film coating material is hydroxypropyl methylcellulose) 100.0g, set aside.
[0079] Take diclofenac sodium, micropowder silica gel, and talcum powder, pass through an 80-mesh sieve and mix until uniform, add calcium hydrogen phosphate, hydroxypropyl methylcellulose, and methylcellulose, mix for 30 minutes, then add magnesium stearate, mix for 5 minutes, and press into tablets 10,000 diclofenac sodium sustained-release tablets were prepared after coating with Opadry, the specification was 0.36g, and the yield was 98.23%.
Embodiment 2
[0081] formula:
[0082]
[0083]
[0084] Preparation Process:
[0085] Accurately weigh 1000.0 g of diclofenac sodium, 390.5 g of hydroxypropyl methylcellulose, 895.4 g of microcrystalline cellulose, 51.5 g of micropowdered silica gel, 67.3 g of stearic acid, and 159.1 g of polyvinylpyrrolidone, and set aside.
[0086] Take diclofenac sodium and micropowder silica gel and pass through a 100-mesh sieve and mix until uniform, add microcrystalline cellulose, hydroxypropyl methylcellulose, and polyvinylpyrrolidone and mix for 60 minutes, then add stearic acid and mix for 5 minutes, and press into tablets to obtain diclofenac 10,000 sodium sustained-release tablets, the specification is 0.26g, and the yield is 99.14%.
Embodiment 3
[0088] formula:
[0089]
[0090] Preparation Process:
[0091] Accurately weigh 1000.0g of diclofenac sodium, 439.6g of hydroxypropyl methylcellulose, 2459.2g of lactose, 251.7g of micropowdered silica gel, 100.0g of talcum powder, 59.0g of polyvinylpyrrolidone, and 60.0g of Eudragit (the film coating is acrylic resin) ,spare.
[0092] Take diclofenac sodium, micropowder silica gel, and talcum powder and pass through a 120-mesh sieve and mix until uniform, add lactose, hydroxypropyl methylcellulose, and polyvinylpyrrolidone, mix for 10 minutes, press into tablets, and coat with acrylic resin to prepare diclofenac sodium sustained-release 10,000 pieces, the specification is 0.43g, and the yield is 98.47%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com