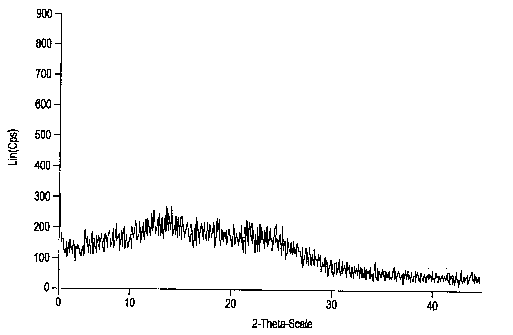
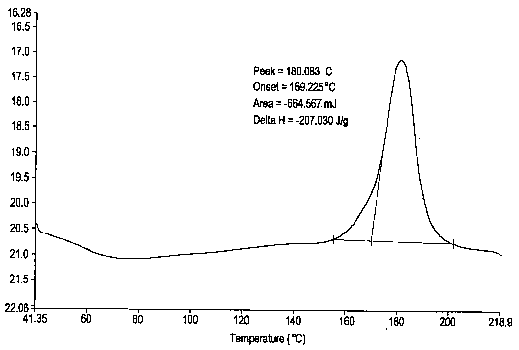
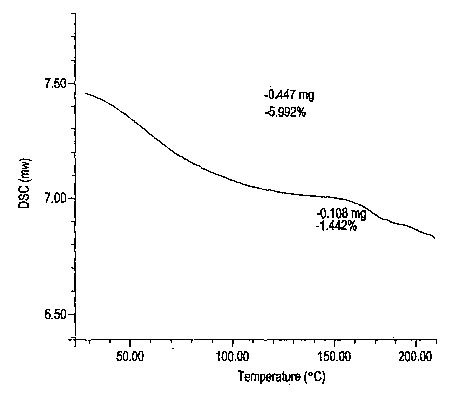
Esomeprazole magnesium trihydrate and preparation method thereof
A technology of esomeprazole magnesium trihydrate and esomeprazole sodium, applied in the field of esomeprazole magnesium trihydrate, can solve the problem of undisclosed esomeprazole magnesium quality data and unstable quality , the rapid growth of impurities and other problems, to achieve the effect of stable properties, high chemical and optical purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1 esomeprazole sodium
[0060] Raw materials: esomeprazole is commercially available, chemical purity 97%, optical purity 98%
[0061] Dissolve 2.6kg (7.54mol) of esomeprazole in 1.2L of ethyl acetate, then add 3.6L of acetone, then add 212.0g (5.28mol) of sodium hydroxide solid, stir and crystallize to obtain esomeprazole Sodium solid 1.564kg, yield 56.5%, chemical purity 99.83%, optical purity 99.91%.
Embodiment 2
[0062] The preparation of embodiment 2 esomeprazole sodium
[0063] Raw materials: esomeprazole is commercially available, chemical purity 97%, optical purity 98%
[0064] Dissolve 2.6kg (7.54mol) of esomeprazole in 1.05L of ethyl acetate, then add 2.6L of acetonitrile, then add 302g (7.54mol) of solid sodium hydroxide, stir and crystallize to obtain esomeprazole sodium The solid is 1.785kg, the yield is 64.5%, the chemical purity is 99.88%, and the optical purity is 99.94%.
Embodiment 3
[0065] The preparation of embodiment 3 esomeprazole sodium
[0066] Raw materials: esomeprazole is commercially available, chemical purity 97%, optical purity 98%
[0067] Dissolve 5.0kg (14.5mol) of esomeprazole in 2.5L of methyl isobutyl ketone, then add 11.0L of acetonitrile, then add 522g (13.05mol) of sodium hydroxide solid, stir and crystallize to obtain esomeprazole Sodium azole solid 4.05kg, yield 74.5%, chemical purity 99.90%, optical purity 99.92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com