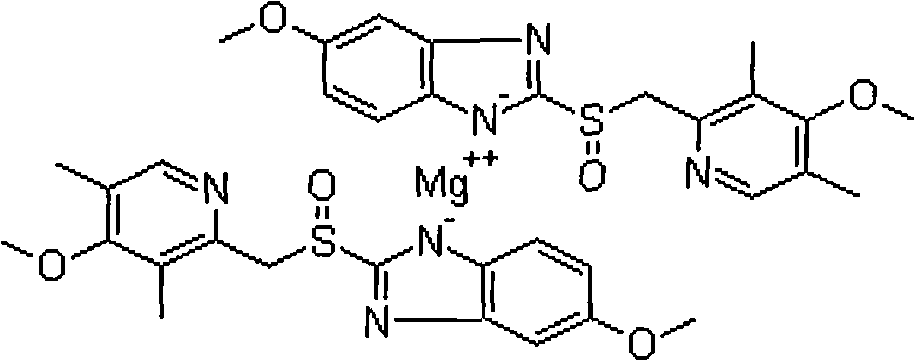
Esomeprazole magnesium sustained-release dropping pill and preparation method thereof
A technology of esomeprazole magnesium and sustained-release dripping pills, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of decreased bioavailability and out-of-control drug release time , increase in drug-related substances, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] First group:
[0033] Based on the total weight of 100g, weigh the base PEG4000 40%, PEG6000 10%, PEG10000 10%, stearic acid 11%, glyceryl monostearate 17%, stabilizer vitamin E 2%, raw material esomeprazole magnesium 10%; heat the substrate in a heating container and stir to melt it, add the corresponding proportion of esomeprazole magnesium, fully stir, and then add the stabilizer vitamin E and stir evenly, under the condition of heat preservation, it will melt or The mixed liquid medicine is dropped into a condensation column containing dimethyl silicone oil, where the temperature during heating and melting is 55°C, the temperature at the upper part of the condensate is 20°C, and the temperature at the bottom is -4°C; take it out after forming.
[0034] The resulting product has a cumulative release percentage of 33 to 56% in 2 hours, 61 to 83% in 6 hours, and 72 to 93% in 10 hours. There is no significant change in the assessment of related substances for 3 consecutive ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com