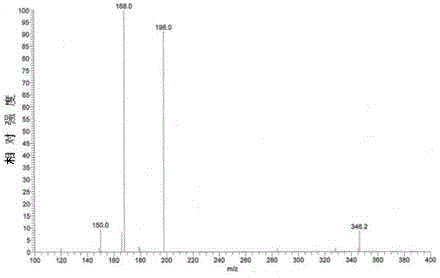
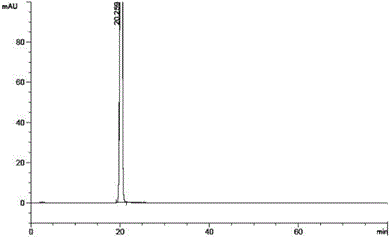
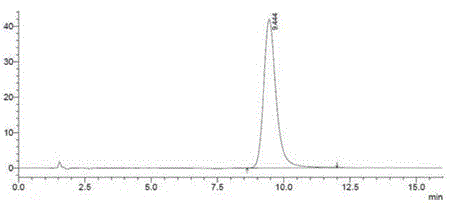
Preparation method of esomeprazole magnesium
A technology of esomeprazole magnesium and magnesium methoxide, which is applied in organic chemistry and other fields, can solve the problems that the final product is difficult to meet the quality standard, is not easy to dry, and has poor stability, and achieves simple and feasible preparation operations, avoiding a large amount of heat release, pH The effect of value stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Disperse 100g of 5-methoxy-2-mercaptobenzimidazole in 500ml of dichloromethane, lower the temperature to 0~5℃, and add 44ml of 15wt% sodium hydroxide aqueous solution to the above system, and the addition is complete. Afterwards, control the temperature to 0~5℃, and continue to add dropwise a solution of 125g 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 125ml water. After the addition is complete, The temperature was raised to reflux and reacted for 2 hours, then the temperature was lowered to 15°C, and the mixture was allowed to stand for liquid separation. 200ml of water was added to the dichloromethane phase, stirred for 15 minutes, and the dichloromethane phase was obtained by standing for liquid separation.
[0029] 2) Add 40g of titanium tetraisopropoxide and 60g of D-diethyl tartrate to the dichloromethane phase, heat up to reflux, react for 1h, cool to 15°C, add 20g of diisopropylethylamine, and control the temperature from -20 to -15 At ℃, ad...
Embodiment 2
[0032] 1) Disperse 50g of 5-methoxy-2-mercaptobenzimidazole in 200ml of n-hexane, lower the temperature to -5~0°C, and add 30g of 20wt% potassium hydroxide aqueous solution to the above system. Then, control the temperature to 0~5℃, add dropwise a solution of 70g 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 100ml water under vigorous stirring, and the addition is complete Afterwards, the temperature was raised to reflux and reacted for 3 hours, and then the temperature was lowered to 25° C., and the mixture was allowed to stand for liquid separation. 200 ml of water was added to the n-hexane phase, stirred for 15 minutes, and then stood for liquid separation to obtain the n-hexane phase.
[0033] 2) Add 20.2g of titanium tetraisopropoxide and 29.8g of D-diethyl tartrate to the n-hexane phase, heat to reflux, react for 3h, cool to 20℃, add 7.5g of triethylamine, control the temperature at -20~-15℃ , The solution prepared by 87.6g 80wt% cumene hydroperoxide and 9...
Embodiment 3
[0036] 1) Disperse 100g of 5-methoxy-2-mercaptobenzimidazole in 500ml of dichloromethane, cool to 0~5℃, add dropwise 44g of 15wt% sodium hydroxide aqueous solution to the above system, and the addition is complete Then, control the temperature to 0~5℃, and continue to add dropwise a solution made of 140g 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and 150ml water. After the addition is complete, The temperature was raised to reflux and reacted for 2 hours, then the temperature was lowered to 15°C, and the mixture was allowed to stand for liquid separation. Add 200 ml of water to the organic phase, stir for 15 minutes, and stand for liquid separation to obtain the methylene chloride phase.
[0037] 2) Add 38.5g of titanium tetraisopropoxide and 62.3g of D-diethyl tartrate to the dichloromethane phase, increase the temperature to reflux, react for 1h, reduce the temperature to 20℃, add 15.2g of triethylamine, and control the temperature from -20~-15 At °C, a solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com