Preparation method of esomeprazole and magnesium salt thereof
A technology of esomeprazole and magnesium salt, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of less application and instability, and achieve the effects of improving the reaction system, being easy to realize, and improving oxidation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
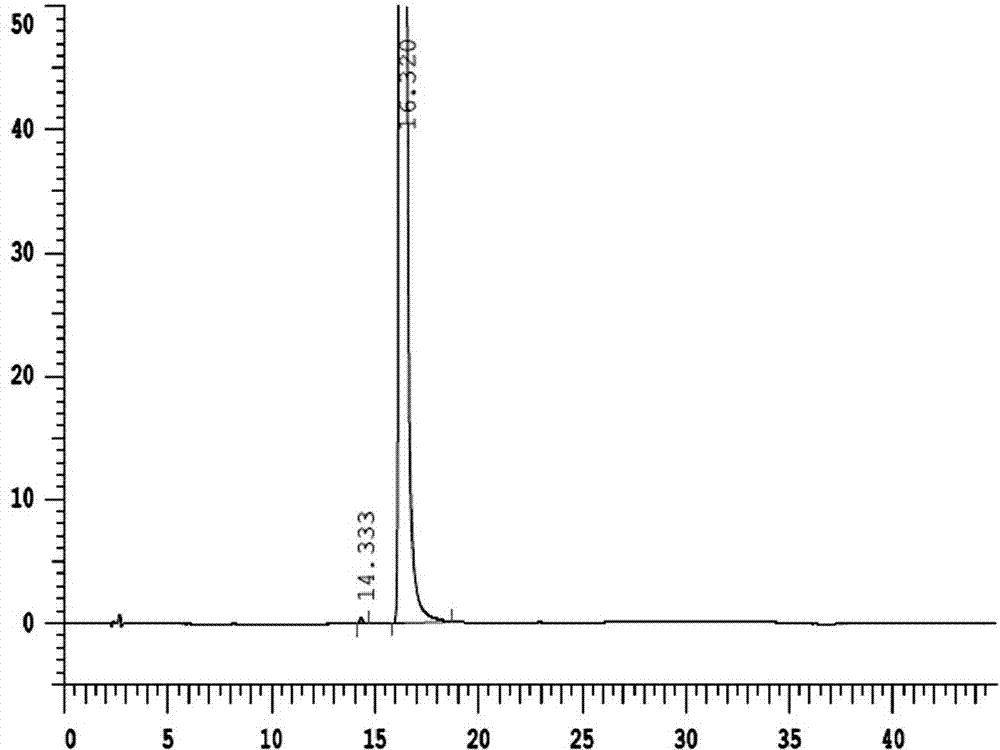
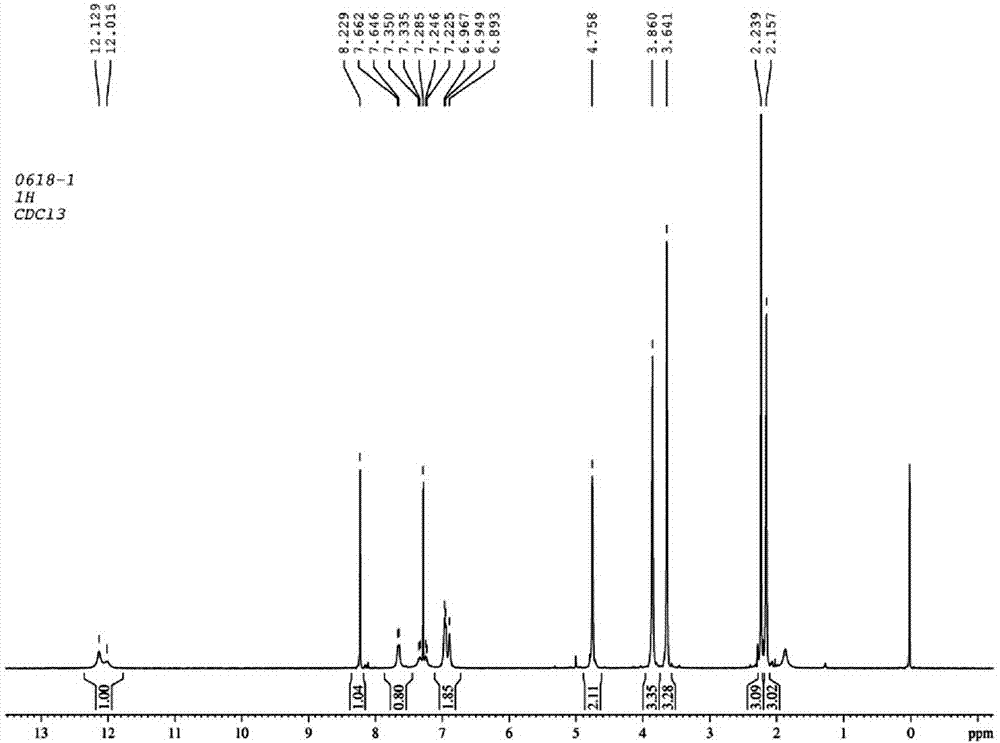
Image
Examples
Embodiment 1
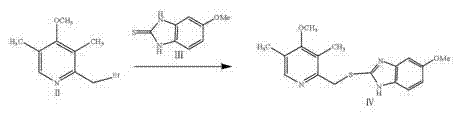
[0042] b. Add methanol to the obtained compound II, the amount of methanol added is 3 times the mass of compound II, add methanol and stir and mix evenly to obtain compound II methanol solution;
[0043] Add methanol and sodium hydroxide solution to 5-methoxy-2-mercaptobenzimidazole, compound III, stir and mix evenly to obtain a compound III solution; the amount of methanol added is 3 times the mass of compound III, and the hydroxide The amount of sodium added is twice the molar number of compound III; the sodium hydroxide is formulated into a solution with a concentration of 50% by mass;
[0044] c. At 25°C, add the compound II methanol solution obtained in step b to the compound III solution dropwise. The volume ratio between the compound III solution and the compound II methanol solution is 1.5:1. Finally, the reaction was carried out at 25°C for 1.5 hours. After the reaction, crystallization, filtration, water washing and drying were carried out in sequence. After drying, ...
Embodiment 2
[0055] In step b: the amount of methanol added is twice the mass of compound II;
[0056] The amount of methanol added is twice the mass of compound III, and the amount of sodium hydroxide added is 1.5 times the molar number of compound III;
[0057] In step c: at 10°C, the compound II methanol solution obtained in step b is added dropwise to the compound III solution, the volume ratio between the compound III solution and the compound II methanol solution is 1.8:1, dropwise After completion, the reaction was carried out at 10°C, and the reaction time was 2 hours;
[0058] In step d: Stir evenly, raise the temperature to 50°C, keep warm at this temperature for 3h, then cool to room temperature, then add 1,3-propylenediamine, lower the temperature to -5~0°C, then slowly add peroxide After the tert-amyl benzoate and tert-amyl peroxybenzoate are added dropwise, the temperature is raised to 5-10°C for reaction, and the reaction lasts for 1.5 hours. After the reaction, the obtaine...
Embodiment 3
[0063] In step b: the amount of methanol added is 4 times the mass of compound II;
[0064] Add methanol and potassium hydroxide solution to 5-methoxy-2-mercaptobenzimidazole, i.e. compound III, the amount of methanol added is 4 times the mass of compound III, and the amount of potassium hydroxide added is the number of moles of compound III 3 times;
[0065] In step c: at 30°C, add the compound II methanol solution obtained in step b to the compound III solution dropwise, the volume ratio between the compound III solution and the compound II methanol solution is 1.2:1, drop After the addition, the reaction was carried out at 30°C, and the reaction time was 0.5h;
[0066] In step d: Stir evenly, raise the temperature to 70°C, keep warm at this temperature for 1.5h, then cool to room temperature, then add methylamine, lower the temperature to -15~-10°C, then slowly add disuccinic acid peroxide dropwise , After adding disuccinic acid peroxide dropwise, the temperature was rais...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com