A kind of preparation method of esomeprazole magnesium
A technology of esomeprazole magnesium and azole thioether, which is applied in the field of pharmaceutical preparation and can solve problems such as inconvenient operation, being caught in it, and difficult to remove impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
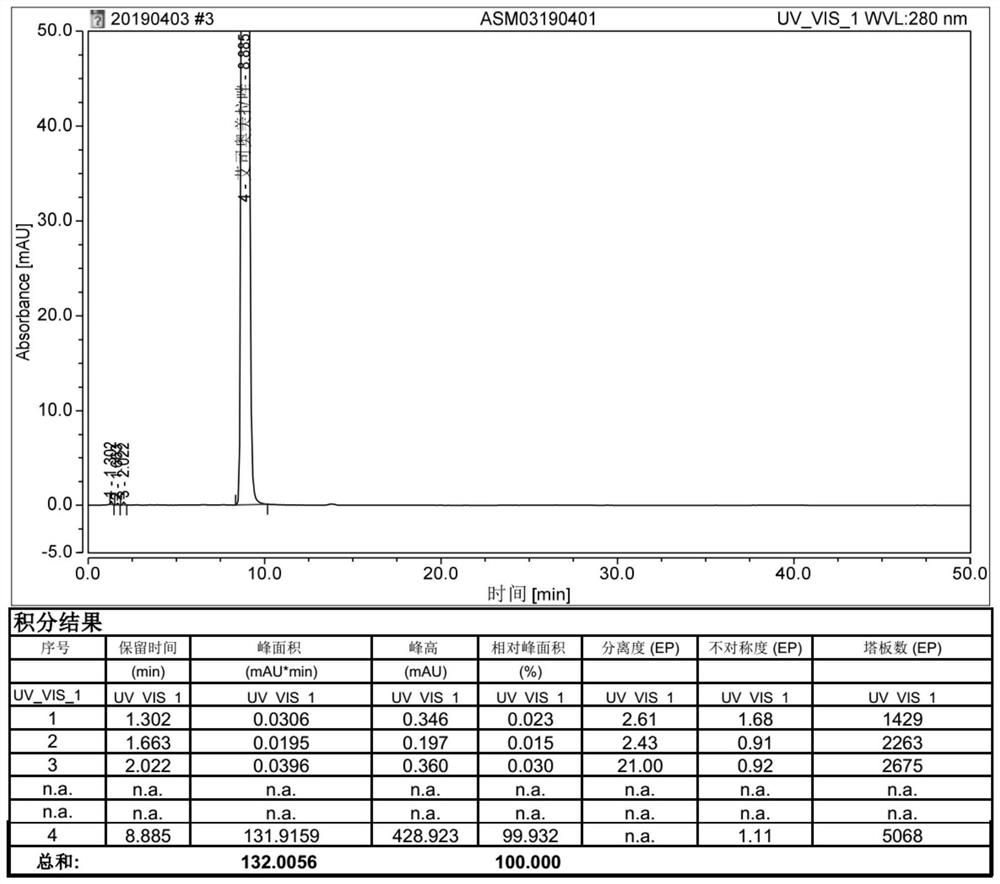
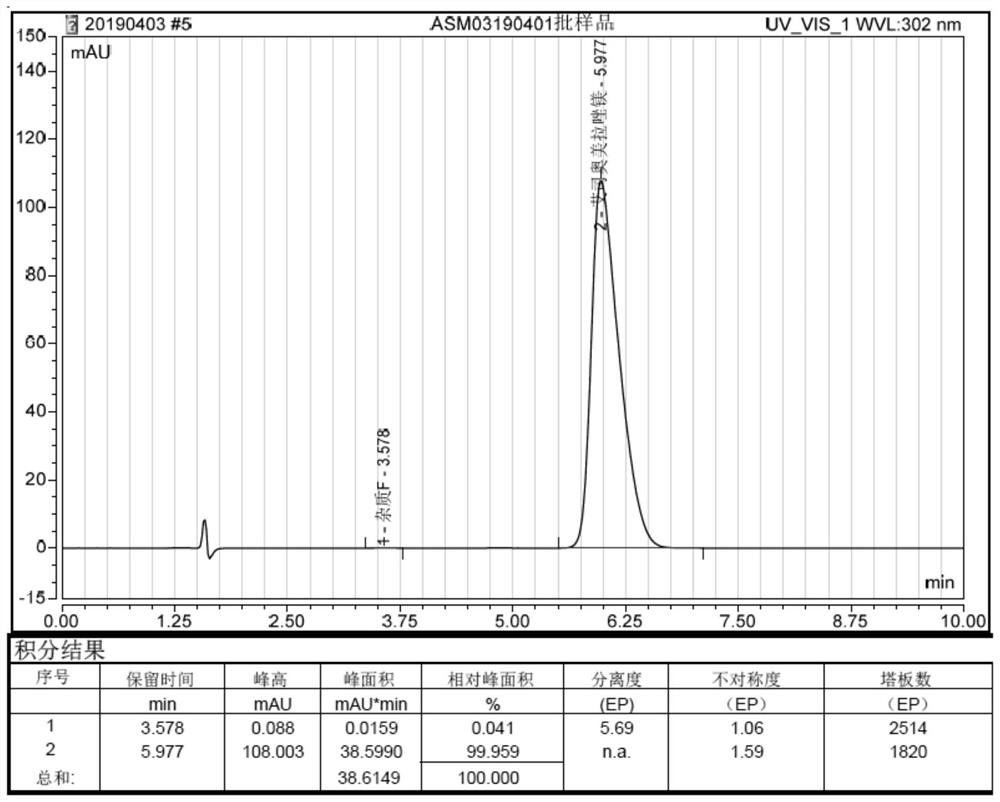
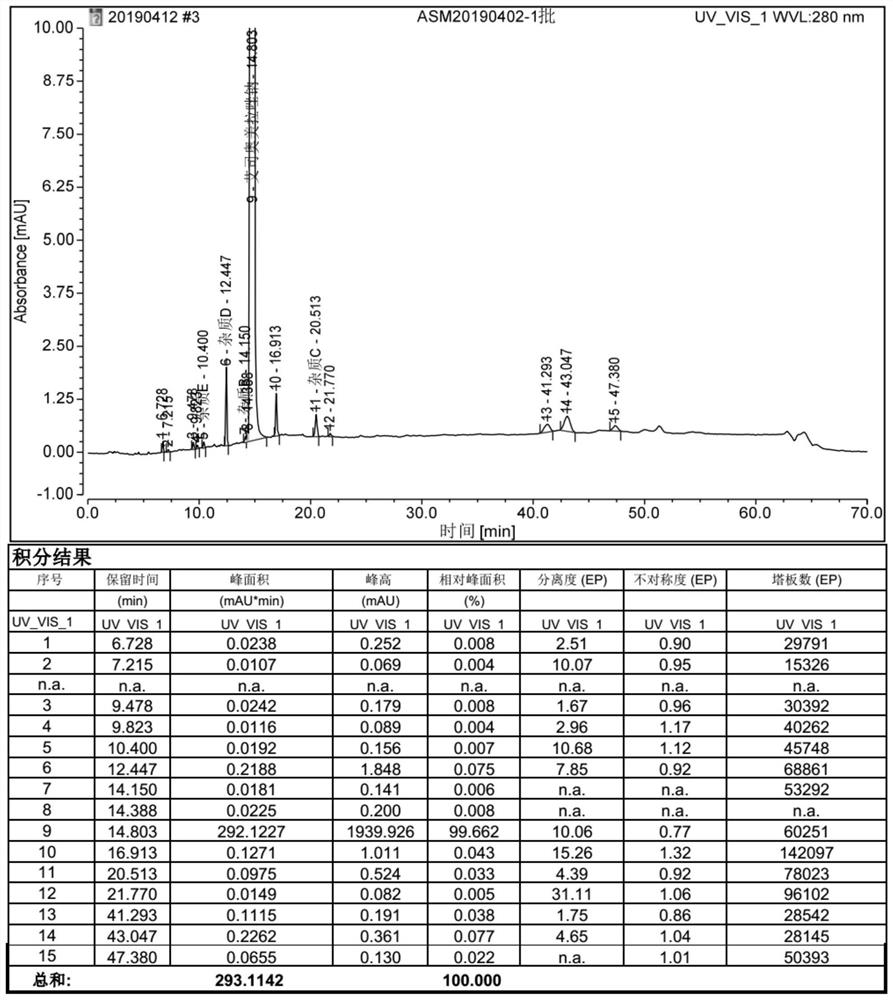
Image
Examples
Embodiment 1
[0036] Preparation of sodium salt: Weigh 10 g (55.48 mmol) of 2-mercapto-5-methoxybenzimidazole into 100 ml of 5% aqueous sodium hydroxide solution, stir to dissolve. Take 12.5g (56.28mmol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and dissolve it in 120ml of dichloromethane, add it dropwise, heat up to reflux, keep at reflux for 1 hour, Sample TLC. After the reaction was completed, the liquid was separated, the organic phase was washed with 50 ml of purified water, the liquid was separated, the organic phase was dried with 10 g of anhydrous sodium sulfate, and filtered.
[0037] The organic phase was transferred into a 500ml three-necked flask, heated to slight reflux, and then 3.8g of diethyl tartrate was added, stirred well, then 2.6g of isopropyl titanate was added dropwise, and stirred for 0.5h. Cool down to 10±2°C, add 1.2g of N,N`-diisopropylethylamine, stir to dissolve, add dropwise 4.8g of cumene hydroperoxide, react for 2 hours, sample for TLC, ...
Embodiment 2
[0040] Preparation of sodium salt: Weigh 10 g (55.48 mmol) of 2-mercapto-5-methoxybenzimidazole into 100 ml of 5% aqueous sodium hydroxide solution, stir to dissolve. Take 12.5g (56.28mmol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride and dissolve it in 120ml of toluene, add dropwise, heat up to reflux, keep at reflux for 1 hour, and sample for TLC . After the reaction was completed, the liquid was separated, the organic phase was washed with 50 ml of purified water, the liquid was separated, the organic phase was dried with 10 g of anhydrous sodium sulfate, and filtered. The organic phase was transferred into a 500ml three-necked flask, and after the temperature was raised to 55°C, 3.8g of diethyl tartrate was added, stirred well, then 2.6g of isopropyl titanate was added dropwise, and stirred for 0.5h. Cool down to 10±2°C, add 1.2g of N,N`-diisopropylethylamine, stir to dissolve, add dropwise 4.8g of cumene hydroperoxide, react for 2 hours, sample for TLC,...
Embodiment 3
[0044] Preparation of sodium salt: Weigh 10g of 2-mercapto-5-methoxybenzimidazole and add it to 100ml of 5% potassium hydroxide aqueous solution, stir to dissolve. Dissolve 12.5 g of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride in 120 ml of toluene, add dropwise, heat up to reflux, keep at reflux for 1 hour, and sample for TLC. After the reaction was completed, the liquid was separated, the organic phase was washed with 50 ml of purified water, the liquid was separated, the organic phase was dried with 10 g of anhydrous sodium sulfate, and filtered.
[0045] The organic phase was transferred into a 500ml three-necked flask, heated to slight reflux, and then 3.8g of diethyl tartrate was added, stirred well, then 2.6g of isopropyl titanate was added dropwise, and stirred for 0.5h. Cool down to 10±2°C, add 1.2g of N,N`-diisopropylethylamine, stir to dissolve, add dropwise 4.8g of cumene hydroperoxide, react for 2 hours, sample for TLC, finish the reaction with 5% s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com