Method for synthesizing diastereoisomer of Doranidazole intermediate
A technology of diastereoisomers and synthetic methods, which is applied in the direction of organic chemistry, can solve the problems of easy explosion, unsatisfactory operation yield and purity, instability, etc., and achieve the safety of raw materials and reagents, easy operation and large-scale production , to avoid the effect of hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
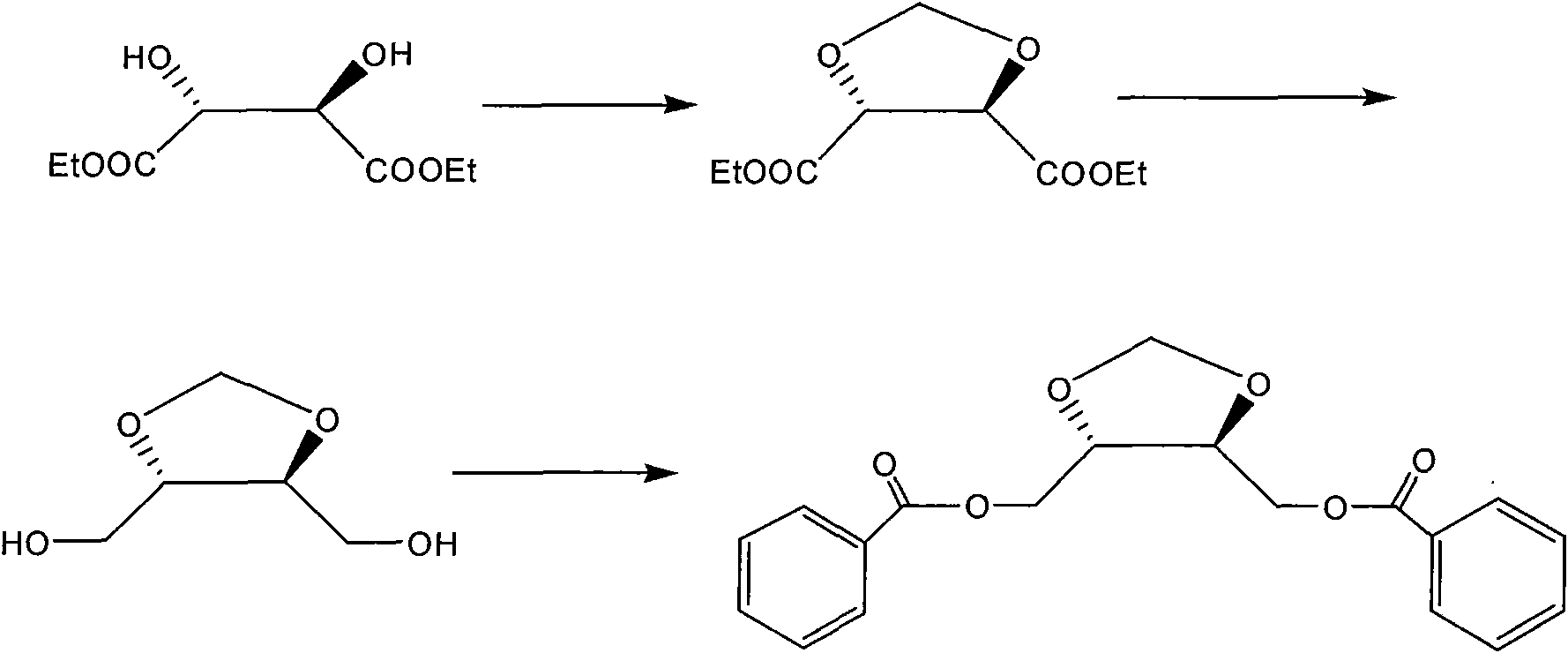
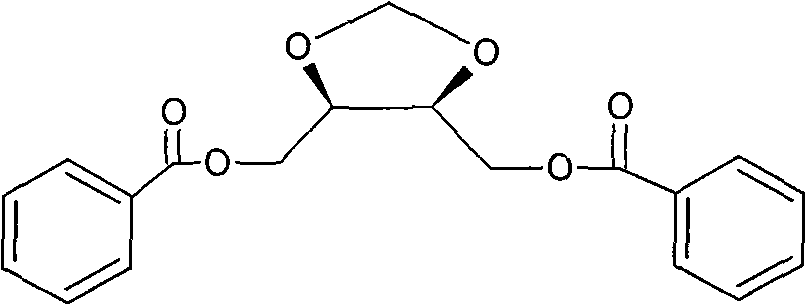
[0024] Synthesis of (4R, 5R)-4,5-bis(benzoyloxymethyl)-1,3-dioxolane a) (4R,5R)-4,5-bis(ethoxy Preparation of carbonyl)-1,3-dioxolane
[0025] Add 100g of L-diethyl tartrate, 100g of anhydrous calcium chloride, 50g of paraformaldehyde into the reactor, add 1000ml of toluene, then add 50ml of boron trifluoride ether, heat and stir, stir at 78-82°C for 3 hours, cool To 0-10°C, add triethylamine dropwise to the reaction solution until the pH is 9, filter, and concentrate the filtrate to obtain an oily substance. Purification by silica gel column chromatography (elution with ethyl acetate / petroleum ether 1:20) gave 30.1 g of the product.
[0026] b) Preparation of (4R, 5R)-4,5-bis(hydroxymethyl)-1,3-dioxolane
[0027] Add 5.94 g of lithium aluminum hydride into 70 ml of THF in portions, and dissolve 30.1 g of (4R,5R)-4,5-bis(ethoxycarbonyl)-1,3-dioxolane obtained in a) in 23 ml of THF, And dropwise added to the aforementioned mixture of lithium aluminum hydride and THF, first s...
Embodiment 2
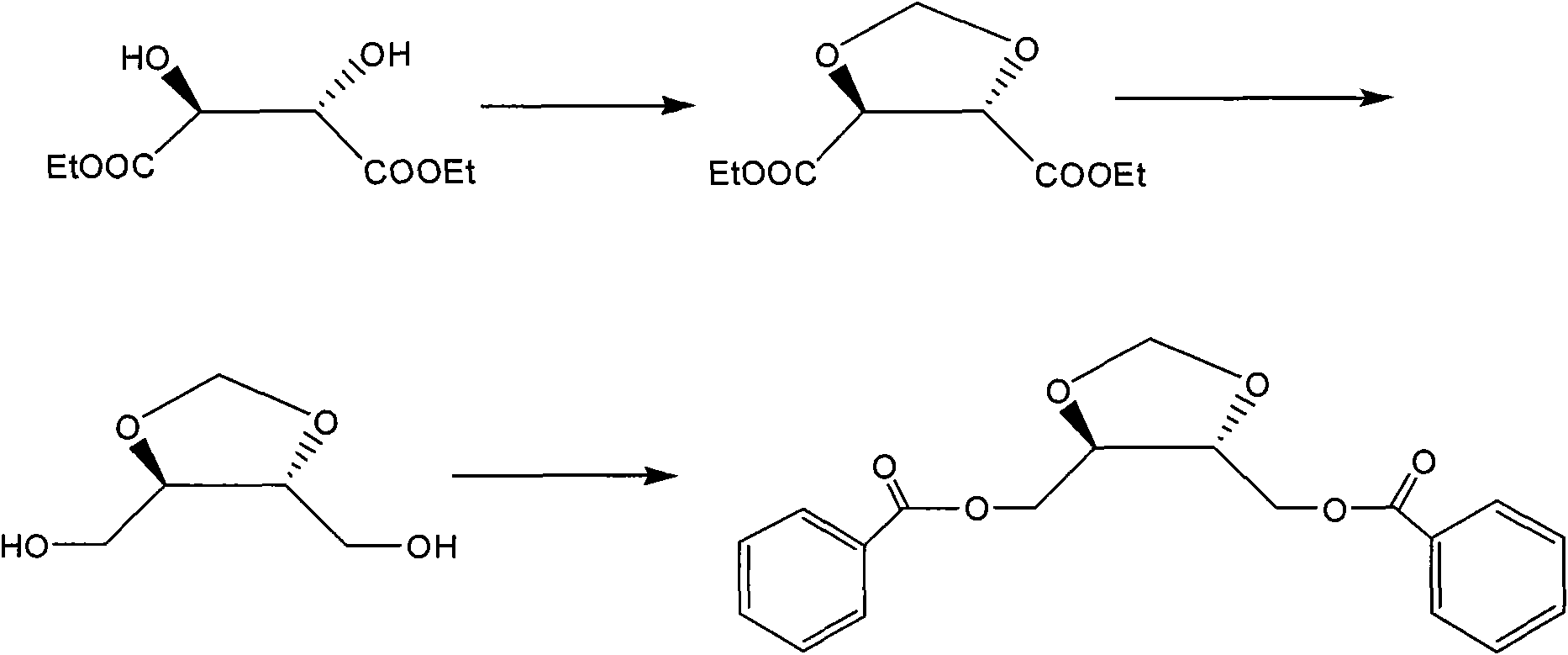
[0036] Embodiment 2 (4S, 5S)-4, the synthesis of 5-bis(benzoyloxymethyl)-1,3-dioxolane
[0037] a) Preparation of (4S, 5S)-4,5-bis(ethoxycarbonyl)-1,3-dioxolane
[0038] Add 100g of D-diethyl tartrate, 100g of anhydrous calcium chloride, and 50g of paraformaldehyde into the reactor, add 1000ml of benzene, then add 50ml of boron trifluoride ether, heat and stir, and react with stirring at 78-82°C for 3 hours. Cool to 0-10°C, add triethylamine dropwise to the reaction solution until the pH is 9, filter, and concentrate the filtrate to obtain an oil. Purification by silica gel column chromatography (elution with ethyl acetate / petroleum ether 1:20) gave 30 g of the product.
[0039] b) Preparation of (4S, 5S)-4,5-bis(hydroxymethyl)-1,3-dioxolane
[0040]Add 5.92 g of lithium aluminum hydride into 70 ml of THF in portions, and dissolve 30 g of (4S, 5S)-4,5-bis(ethoxycarbonyl)-1,3-dioxolane obtained in the above a) in 23 ml of THF, And dropwise added to the above-mentioned mixtur...
Embodiment 3
[0049] Embodiment 3 (4S, 5S)-4, the synthesis of 5-bis(benzoyloxymethyl)-1,3-dioxolane
[0050] a) Preparation of (4S, 5S)-4,5-bis(ethoxycarbonyl)-1,3-dioxolane
[0051] Add 100g of D-diethyl tartrate, 100g of anhydrous calcium chloride, and 50g of paraformaldehyde into the reactor, add 1000ml of toluene, then add 50ml of boron trifluoride ether, heat and stir, and react with stirring at 78-82°C for 2 hours, Cool to 0-10°C, add triethylamine dropwise to the reaction solution until the pH is 9, filter, and concentrate the filtrate to obtain an oil. Purification by silica gel column chromatography (elution with ethyl acetate / petroleum ether 1:20) yielded 31.2 g of the product.
[0052] b) Preparation of (4S, 5S)-4,5-bis(hydroxymethyl)-1,3-dioxolane
[0053] Add 6.00 g of lithium aluminum hydride into 70 ml of THF in portions, and dissolve 31.2 g of (4S, 5S)-4,5-bis(ethoxycarbonyl)-1,3-dioxolane obtained in a) in 25 ml of THF, And dropwise added to the above-mentioned mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com