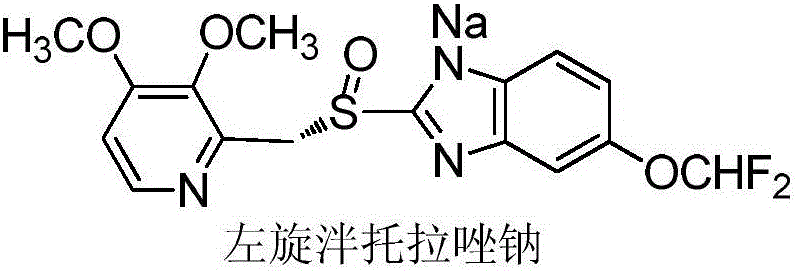
Method for preparing L-pantoprazole sodium
A technology of levorotatory pantoprazole sodium and pantoprazole, applied in the field of medicine, can solve the problems of low yield and high oxidation reaction temperature, and achieve the effects of reducing environmental pollution, reducing environmental pollution and improving labor protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 1.04 kg of toluene, 200 g of pantoprazole sulfide, 68 g of D-(-)-diethyl tartrate, and 46 g of tetraisopropyl titanate into the reaction bottle, and after the addition, raise the temperature to 65-70°C and stir for 30 minutes to react slowly. 1.5 g of purified water was added dropwise, and the reaction was stirred for 1 h after the drop was completed. The temperature of the reaction solution was lowered to 20-25°C, 20.8 g of N,N-diisopropylethylamine was added, and stirred for 30 minutes. Cool the solution to -5-0°C, add 288 g of dicumyl hydroperoxide dropwise, keep stirring for 13 hours, and stop the reaction. The reaction solution was extracted three times with 1000 g, 400 g, and 400 g of 7.5% by weight sodium hydroxide solution, and all the aqueous layers were combined. The aqueous layer was adjusted to pH 8.5-9.0 with glacial acetic acid, extracted three times with ethyl acetate, each time 720g, combined ethyl acetate layer, concentrated under reduced pressure ...
Embodiment 2
[0046] Add 5.2kg of toluene, 1.0kg of pantoprazole sulfide, 0.34kg of D-(-)-diethyl tartrate, and 0.23kg of tetraisopropyl titanate into a 20L reaction kettle, heat up to 65-70°C and stir after adding After 40 minutes of reaction, 7.4 g of purified water was slowly added dropwise, and after the completion of the dropwise reaction, the reaction was carried out with insulation and stirring for 1 hour. Cool the reaction solution to 20-25°C, add 0.1 kg of N,N-diisopropylethylamine, and stir for 45 minutes. Cool the solution to -5-0°C, add 1.44 kg of dicumyl hydroperoxide dropwise, keep stirring for 14 hours, and stop the reaction. The reaction solution was extracted three times with 5 kg, 2 kg, and 2 kg of 7.5% by weight sodium hydroxide solution, and all aqueous layers were combined. The aqueous layer was adjusted to pH 8.5-9.0 with glacial acetic acid, extracted three times with ethyl acetate, 3.6 kg each time, combined ethyl acetate layer, concentrated under reduced pressure a...
Embodiment 3
[0049] Add 15.6kg of toluene, 3.0kg of pantoprazole sulfide, 1.02kg of D-(-)-diethyl tartrate, and 0.69kg of tetraisopropyl titanate into a 50L reactor, and heat up to 65-70°C and stir after adding After 40 minutes of reaction, 22.2 g of purified water was slowly added dropwise, and the mixture was stirred and reacted for 1 hour. Cool the reaction solution to 15-20°C, add 0.31 kg of N,N-diisopropylethylamine, and stir for 45 minutes. Cool the solution to -5~0°C, add 4.32 kg of dicumyl hydroperoxide dropwise, keep stirring for 14 hours, and stop the reaction. The reaction solution was extracted three times with 15kg, 6kg, and 6kg of 7.5% by weight sodium hydroxide solution respectively, and all the aqueous layers were combined. The aqueous layer was adjusted to pH 8.0-8.5 with glacial acetic acid, extracted three times with ethyl acetate, 10.8 kg each time, combined ethyl acetate layer, concentrated under reduced pressure at 40-45°C until solids precipitated, and separated at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com