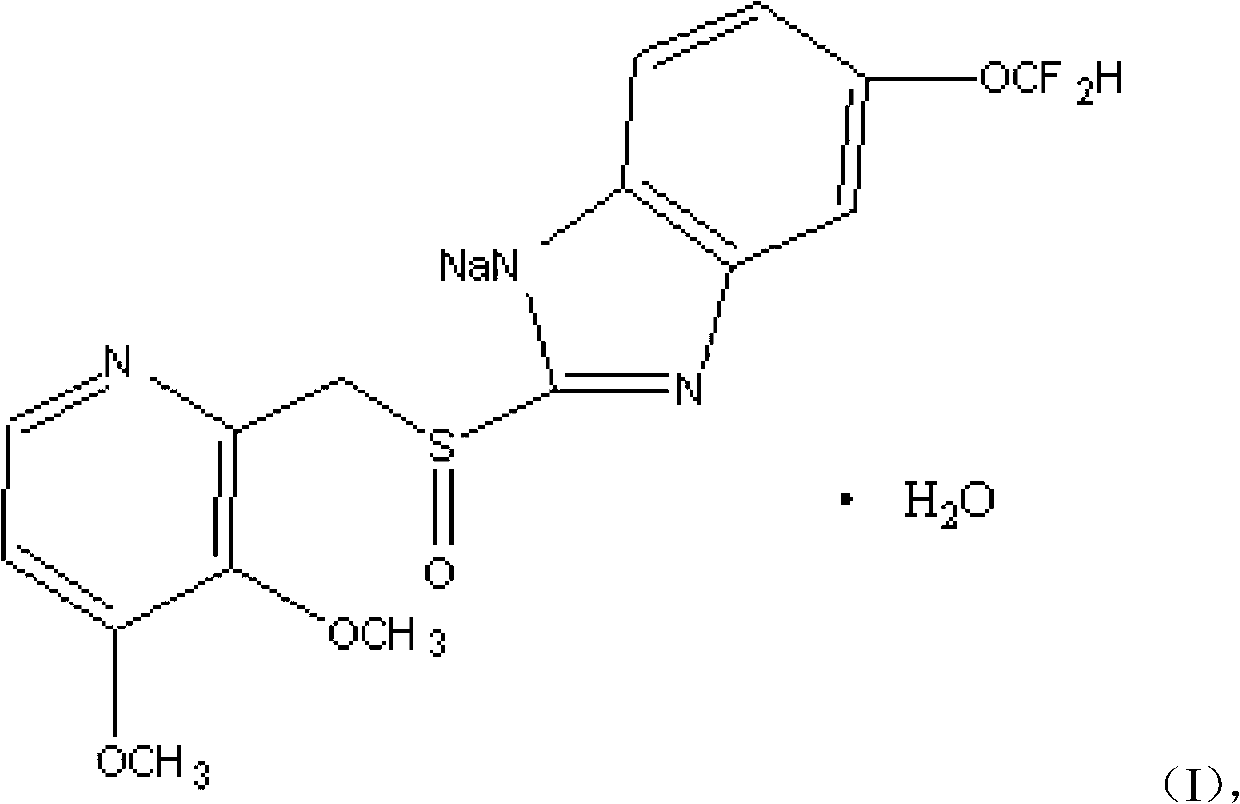
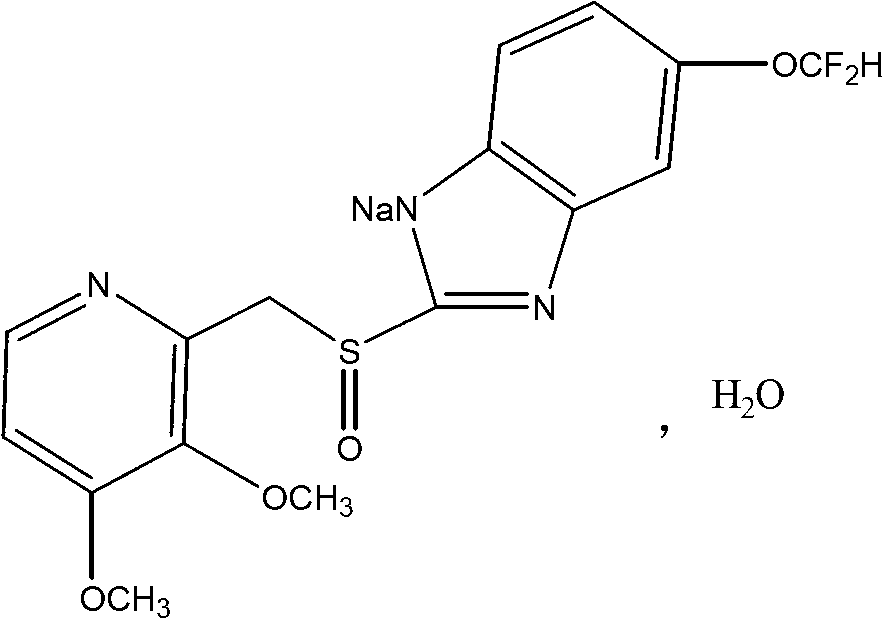
Pantoprazole compound, preparation methods and pharmaceutical preparations thereof
A technology for pantoprazole and pharmaceutical preparations, applied in the field of pharmaceutical compounds, can solve problems such as discoloration and polymerization, and achieve the effects of stable water content, low impurities and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Get 100 grams of crude pantoprazole sodium salt and dissolve it in 600ml of acetone solution with a volume fraction of 98%, and heat the solution to 55°C; then add 1g of activated carbon for adsorption and decolorization, and then cool the solution to 35°C, and then carry out suction filtration Concentrate the filtrate to 350ml under reduced pressure, then add 400ml of ethyl acetate and 200ml of chloroform, stir (60 rpm) and cool down to -2°C, stir for 60min until a large amount of crystals are precipitated; Temperature 40°C, -0.05MPa, vacuum dry for 3.5h. The described pantoprazole sodium compound can be obtained.
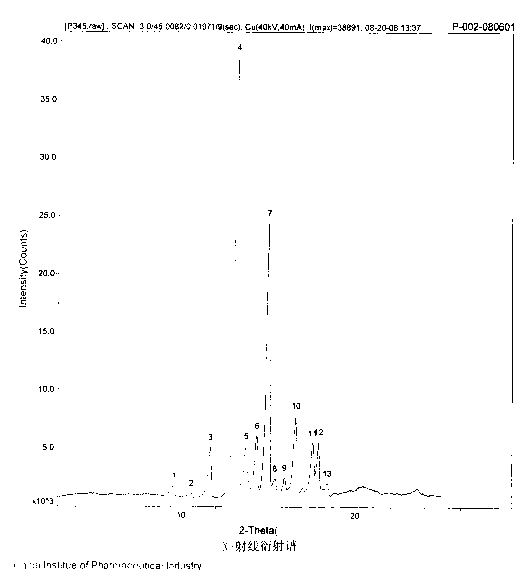
[0041] The obtained pantoprazole sodium compound entity is as follows through X-ray diffraction spectrum figure 1 As shown, it can be seen from the figure that the X-diffraction diagram of the compound entity of the present embodiment is expressed at 9.5 °, 10.4 °, 11.6 °, 13.1 °, 13.8 °, 14.2 °, 15.0 °, 15.3 °, 15.9 ° in the 2θ angle , There are peaks at...
Embodiment 2
[0043] Get 100 grams of crude pantoprazole sodium salt and dissolve it in 650ml of acetone solution with a volume fraction of 98%, and heat the solution to 55°C; then add 1g of activated carbon to carry out adsorption decolorization, and then cool the solution to 38°C, then carry out suction filtration Concentrate the filtrate to 380ml under reduced pressure, then add 400ml of ethyl acetate and 200ml of chloroform, stir (70 rpm) and cool down to 1°C, stir for 60min until a large amount of crystals are precipitated; 43°C, -0.05MPa, vacuum dry for 4h. The described pantoprazole sodium compound entity can be obtained.
[0044] The obtained pantoprazole sodium compound entity has the same melting point and X-ray diffraction spectrum as in Example 1.
Embodiment 3
[0046]Get 100 grams of crude pantoprazole sodium salt and dissolve it in 700 ml of acetone solution with a volume fraction of 98%, and heat the solution to 55°C; then add 1g of activated carbon to carry out adsorption decolorization, and then cool the solution to 40°C, then carry out suction filtration Concentrate the filtrate to 400ml under reduced pressure, then add 400ml of ethyl acetate and 200ml of chloroform, stir (80 rpm) and cool down to 3°C, stir for 60min until a large amount of crystals are precipitated; 45°C, -0.05MPa, vacuum dry for 4h. The described pantoprazole sodium compound entity can be obtained.
[0047] The obtained pantoprazole sodium compound entity has the same melting point and X-ray diffraction spectrum as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com