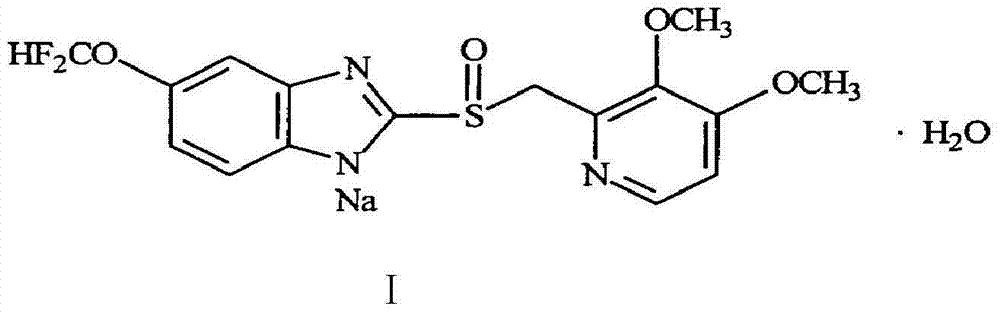
Preparation method of pantoprazole sodium
A technology of pantoprazole sodium and benzimidazole, which is applied in the field of medicine, can solve problems such as cumbersome process, cumbersome post-processing, and environmental impact, and achieve the effects of lowering reaction temperature, easy control of side reactions, and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
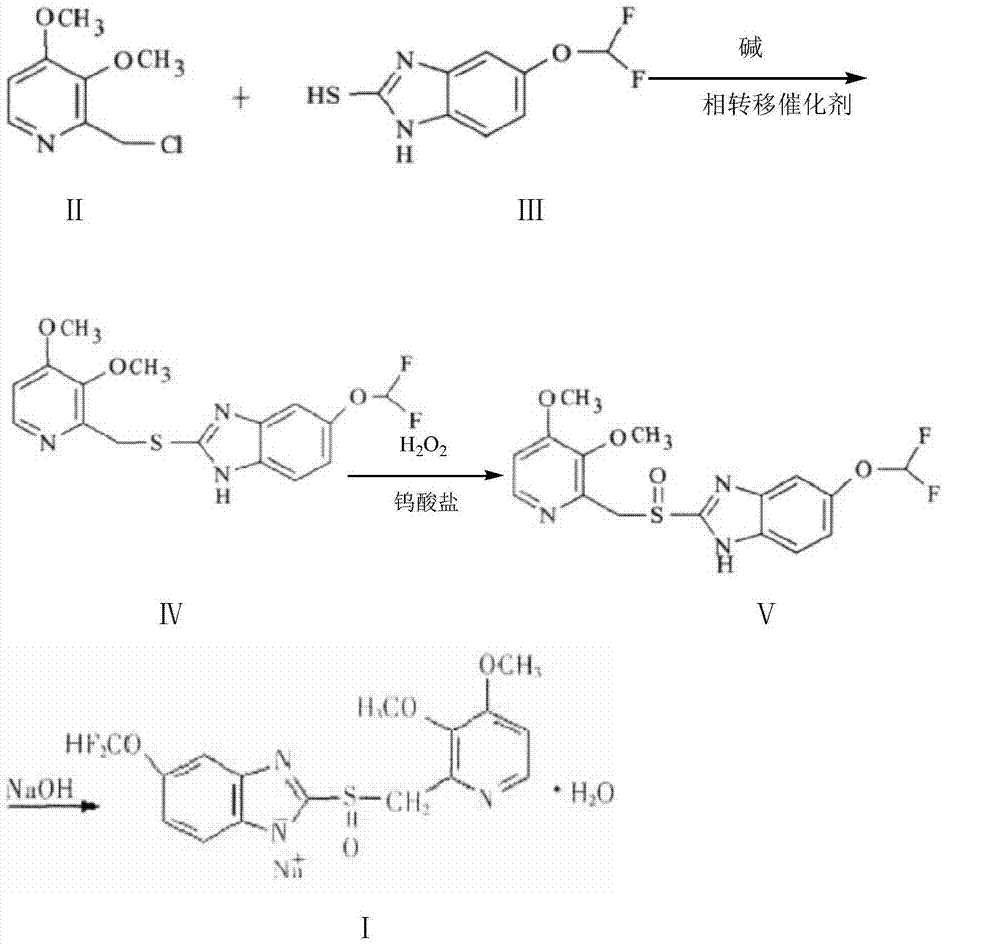
[0029] Add 1L of water, sodium hydroxide (45g, 1.13mol) and triethylbenzylammonium chloride (3g, 0.013mol) into a 2.5L three-necked flask, stir until clear, and add dichloromethane (0.85 L) and 5-difluoromethoxy-2-mercapto-1H-benzimidazole (99.8g, 0.466mol), stir well and drop into 2-chloromethyl 3,4-dimethoxypyridine hydrochloride (99g, 0.44mol) in water (0.6L) solution, dripped within 1.6h, reacted at 30-35°C for 4.5h, TLC [developing solvent: ethyl acetate: methanol = 9: 1] after the completion of the detection reaction, let stand to separate layer, the dichloromethane layer was separated and washed with 0.1mol / L sodium hydroxide solution, and then an aqueous solution of sodium tungstate (Na 2 WO 4 .2H 2 O16g, H2 (00.75L), 250ml of 30% hydrogen peroxide, adjust the pH value to 3.5 with weak acid, react at -6°C-0°C for 4.5h, detect by TLC (developing solvent: ethyl acetate:methanol=9:1), add saturated carbonic acid after the reaction is completed Adjust the pH value of th...
Embodiment 2
[0032] Add 0.95L of water, sodium hydroxide (45.2g, 1.13mol) and tetrabutylammonium bromide (4.83g, 0.015mol) into a 2.5L three-necked flask, stir until clear, cool to room temperature and add dichloromethane (0.85L) and 5-difluoromethoxy-2-mercapto-1H-benzimidazole (99.8g, 0.466mol), stir well and add 2-chloromethyl 3,4-dimethoxypyridinium dropwise Acetate (99g, 0.44mol) in 0.6L of water solution, dripped within 1.6h, reacted at 30-35°C for 4.5h, TLC [developing solvent: ethyl acetate: methanol = 9: 1] after the completion of the detection reaction, let it stand and separate layer, the dichloromethane layer was separated and washed with 0.1mol / L sodium hydroxide solution, and then an aqueous solution of sodium tungstate (Na 2 WO 4 .2H 2 O16.5g, H 2 (00.75L), 255ml of 30% hydrogen peroxide, adjust the pH value to 2.5 with weak acid, react at -5°C-0°C for 4.5h, detect by TLC (developing solvent: ethyl acetate:methanol=9:1), add saturated carbonic acid after the reaction is c...
Embodiment 3
[0035] Add 1L of water, sodium hydroxide (43g, 1.13mol) and tetrabutylammonium bisulfate (5.08g, 0.015mol) into a 2.5L three-necked flask, stir until clear, and add chloroform (0.84L) after cooling to room temperature And 5-difluoromethoxy-2-mercapto-1H-benzimidazole (100g, 0.466mol), after stirring well, drop into 2-chloromethyl 3,4-dimethoxypyridine hydrochloride (98.5g , 0.44mol) of water 0.6L solution, dripped within 1.6h, reacted at 30-35°C for 4.5h, TLC [developing solvent: ethyl acetate: methanol = 9:1] detection, after the completion of the reaction, let it stand for stratification, and separate The chloroform layer was washed with 0.1mol / L sodium hydroxide solution, and then an aqueous solution of potassium tungstate (Na 2 WO 4 .2H 2 O18g, H 2 (00.76L), 30% hydrogen peroxide 260ml, adjusted pH value to 3.5 with weak acid, reacted at -5°C-0°C for 4.5h, detected by TLC (developing solvent: ethyl acetate:methanol=9:1), after the reaction was completed, add saturated c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com