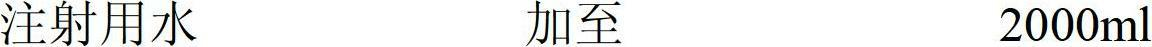S-pantoprazole sodium freeze-drying medicament composition and preparation method thereof
A technology of levo-pantoprazole sodium and its composition, which is applied in the field of levo-pantoprazole sodium freeze-dried pharmaceutical composition and its preparation, and can solve problems such as product quality impact, increased clinical risk, and stability of freeze-dried preparations , to achieve the effect of improving reproducibility and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Prescription:
[0041]
[0042] 2. Preparation process:
[0043]Take water for injection prepared with a total volume of 80%, control the water temperature below 30°C, turn on the agitator, add 1g of disodium ethylenediaminetetraacetate and stir to dissolve, then add 40g of mannitol into the batching tank, stir to dissolve completely, add 1mol / L sodium hydroxide solution to adjust the pH value of the liquid to between 10.0 and 11.0, then add 21.15g of levopantoprazole sodium, stir to dissolve, add water for injection to the full amount, after completely dissolving, add 1g of activated carbon and stir for 15min Decarburization, sampling for pH value and content determination of the intermediate medicinal solution. After the intermediate is qualified, it is filtered, filled and stoppered, and the freeze-drying process is ended when the water content of the finished product is 10-12%, so as to obtain the final freeze-dried preparation.
Embodiment 2
[0045] 1. Prescription:
[0046]
[0047]
[0048] 2. Preparation process:
[0049] Take water for injection prepared with a total volume of 80%, control the water temperature below 30°C, turn on the agitator, add 1.5g of disodium calcium edetate and 20g of mannitol into the batching tank, stir to dissolve completely, add 1mol / L Sodium hydroxide solution adjusts the pH value of the medicinal solution to between 10.0 and 11.0, then add 21.15 g of levopantoprazole sodium, stir to dissolve, add water for injection to the full amount, and after complete dissolution, take a sample for the pH of the intermediate medicinal solution value and content determination. After the intermediate is qualified, it is filtered, filled, and stoppered, and the freeze-drying process is ended when the water content of the finished product is about 15%, to obtain the final freeze-dried preparation.
Embodiment 3
[0051] 1. Prescription:
[0052]
[0053] 2. Preparation process:
[0054] Take water for injection prepared with a total volume of 80%, control the water temperature below 30°C, turn on the agitator, add 0.5g disodium edetate and 70g dextran-40 into the batching tank, stir to dissolve completely, add 1mol / L Sodium carbonate solution adjusts the pH value of the medicinal solution to between 10.0 and 11.0, then add 21.15 g of levo-pantoprazole sodium, stir to dissolve, add water for injection to the full amount, after completely dissolving, add 1 g of activated carbon and stir for 15 minutes to decarburize. Sampling was carried out to determine the pH value and content of the intermediate medicinal solution. After passing the test of the intermediate, it is filtered, filled, and stoppered, and the freeze-drying process is ended when the water content of the finished product is 18-20%, so as to obtain the final freeze-dried preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com