Pharmaceutical composition of pantoprazole sodium and preparation method thereof
A technology of pantoprazole sodium and composition, which is applied in the field of pharmaceutical composition of pantoprazole sodium and its preparation, can solve problems such as unclear product stability, inapplicability to large-scale production, and influence on product stability, To achieve the effect of ensuring the safety of medication, reducing the dosage and speeding up the speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Dissolve pantoprazole sodium solid in distilled water at 40°C, and add a mixed solvent of anhydrous methanol, anhydrous isopropanol and acetone while stirring under a sound field with a frequency of 25KHz and an output power of 30W; among them, The volume ratio of anhydrous methanol, anhydrous isopropanol and acetone is 5:4:1; the volume ratio of the mixed solvent to the solid aqueous solution of pantoprazole sodium is 3.5:1.
[0040] (2) After adding the mixed solvent, lower the temperature to 1°C to obtain crystals and then stand for crystallization; filter, wash the filter cake with absolute ethanol, and vacuum-dry for 6 hours to obtain pantoprazole sodium crystals; the cooling rate is 2.5 °C / min.
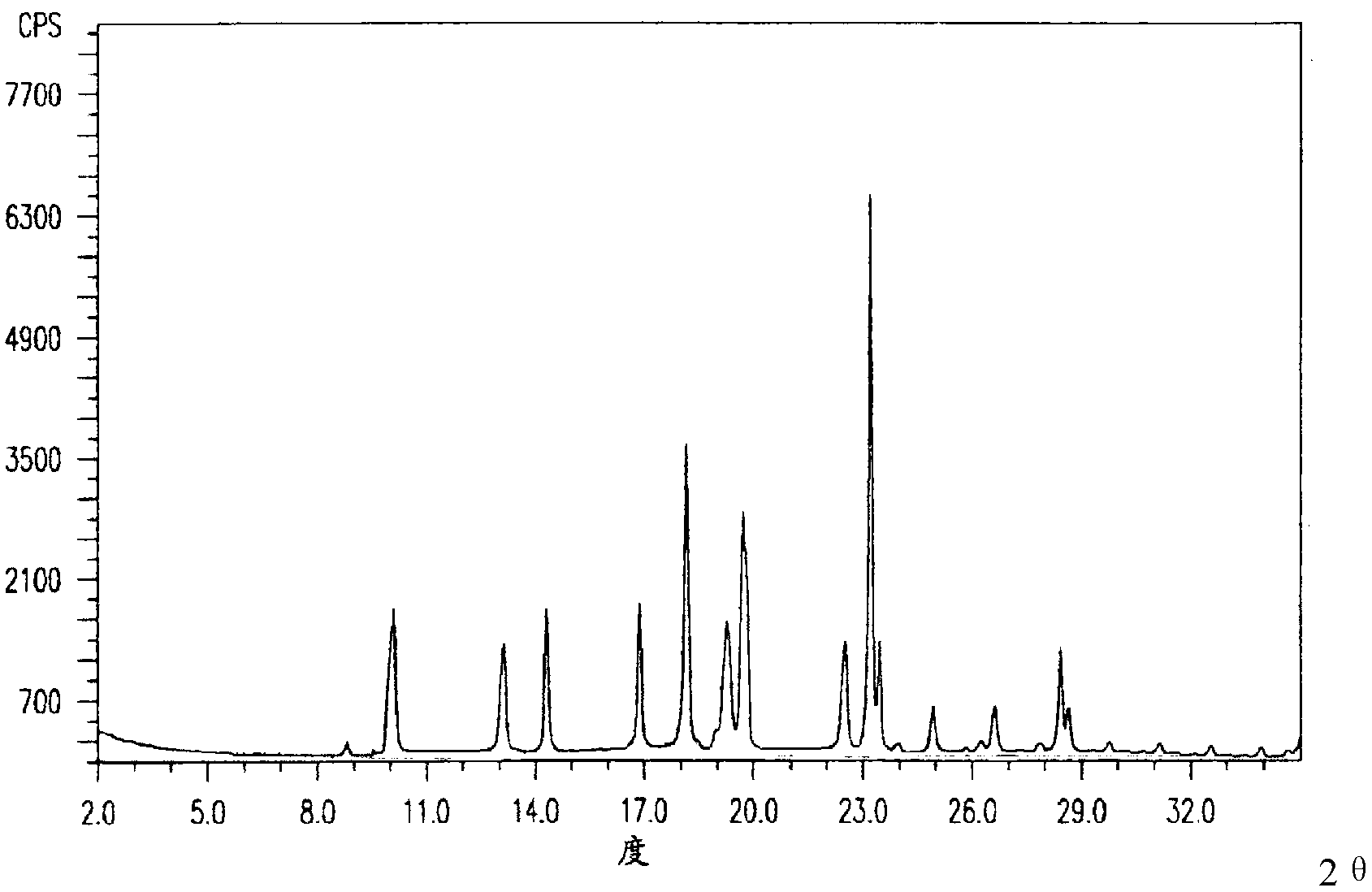
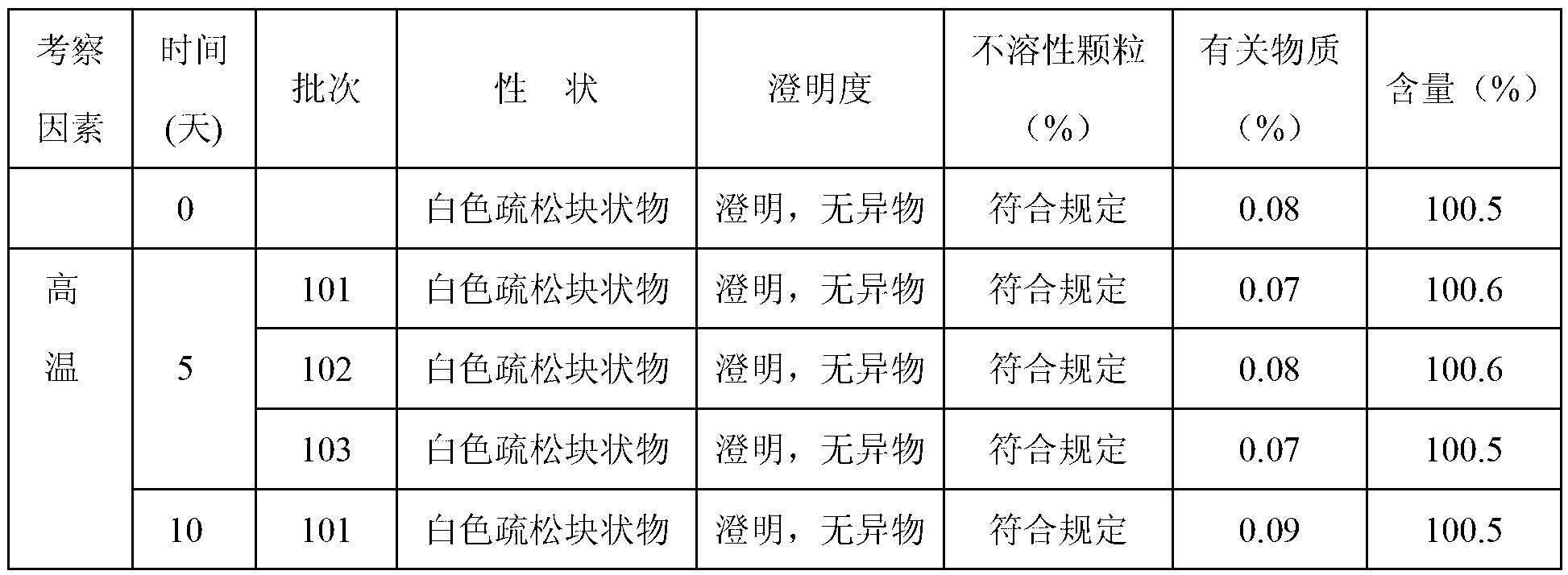
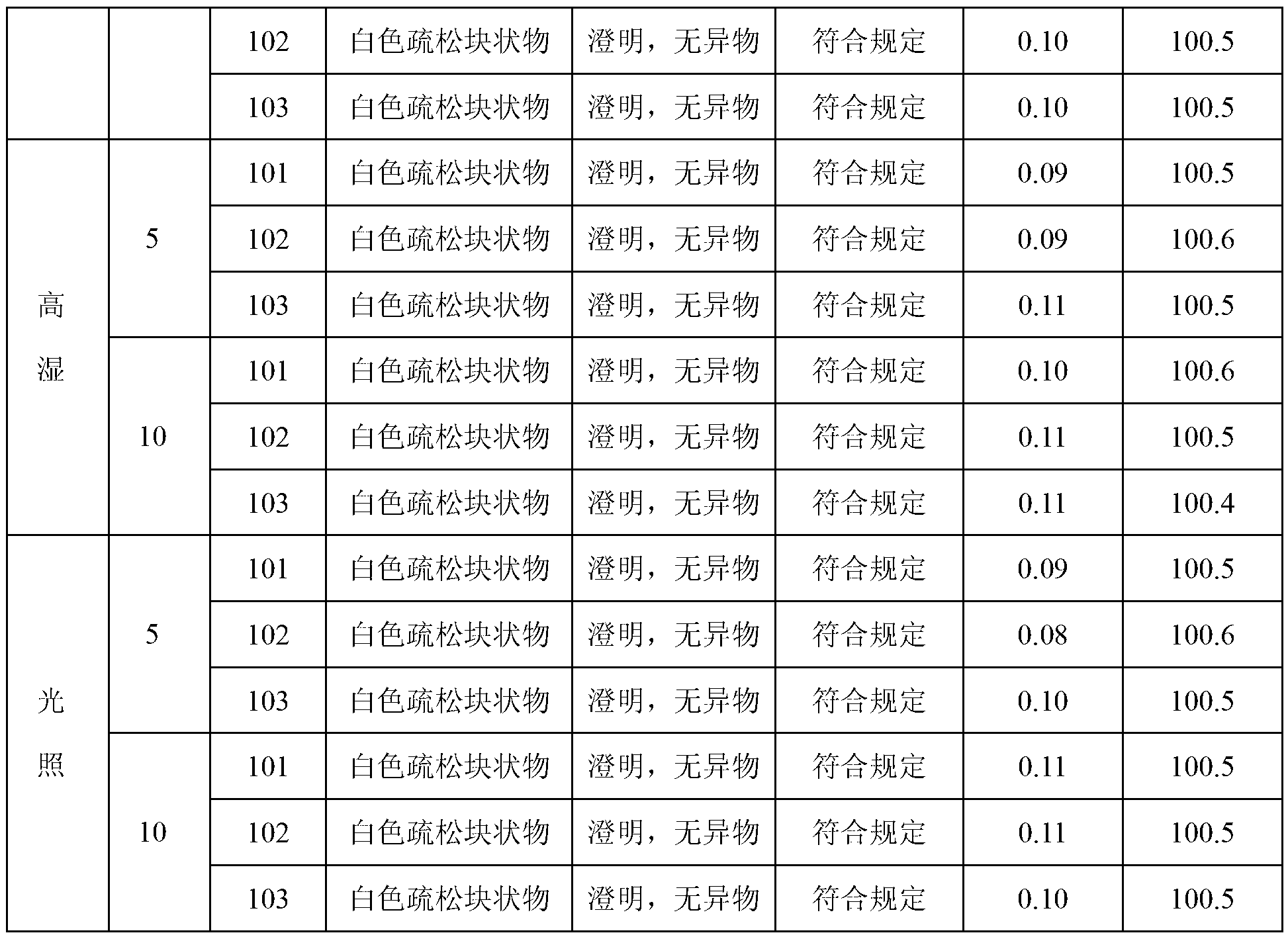
[0041] The X-ray powder diffraction pattern obtained by Cu-Kα ray measurement of the prepared pantoprazole sodium is as follows figure 1 Shown; detected by high performance liquid chromatography, its purity is 99.96%; melting point 203 ~ 205 ℃.
Embodiment 2
[0043] (1) Dissolve pantoprazole sodium solid in distilled water at 45°C, and add a mixed solvent of anhydrous methanol, anhydrous isopropanol and acetone while stirring under a sound field with a frequency of 25KHz and an output power of 60W; anhydrous The volume ratio of methanol, anhydrous isopropanol and acetone is 5:4:2; the volume ratio of the mixed solvent to the solid aqueous solution of pantoprazole sodium is 3:1.
[0044] (2) After adding the mixed solvent, cool down to 2°C to obtain crystals and then stand for crystallization; filter, wash the filter cake with absolute ethanol, and vacuum dry for 8 hours to obtain pantoprazole sodium crystals; the cooling rate is 3.5 °C / min.
[0045] The X-ray powder diffraction pattern obtained by Cu-Kα ray measurement of the prepared pantoprazole sodium is as follows figure 1 Shown; detected by high performance liquid chromatography, its purity is 99.95%; melting point: 203 ~ 205 ℃.
Embodiment 3
[0047] (1) Pantoprazole sodium solid is dissolved in distilled water, under the sound field that frequency is 25KHz, output power is 60W, add the mixed solvent of anhydrous methanol, anhydrous isopropanol and acetone while stirring; Said mixed solvent The volume ratio of pantoprazole sodium solid aqueous solution is 3.25:1.
[0048] (2) After adding the mixed solvent, lower the temperature to 5°C, obtain crystals and then stand for crystallization; filter, wash the filter cake with absolute ethanol, and vacuum-dry for 7 hours to obtain pantoprazole sodium crystals; among them, anhydrous The volume ratio of methanol, anhydrous isopropanol and acetone is 5:4:1.5; the cooling rate is 3°C / min.
[0049] The X-ray powder diffraction pattern obtained by Cu-Kα ray measurement of the prepared pantoprazole sodium is as follows figure 1 Shown; detected by high performance liquid chromatography, its purity is 99.95%; melting point: 203 ~ 205 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com