Pharmaceutical composition of fludarabine phosphate and preparation method thereof
A technology of fludarabine phosphate and its composition, which is applied in the pharmaceutical composition of fludarabine phosphate and its preparation field, and can solve the problems of high impurity content and potential safety hazards of fludarabine phosphate, so as to ensure drug safety Sex, reduce dosage, and ensure the effect of freeze-dried form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Prepare a mixed solvent according to the volume ratio of water: acetone and ethanol is 5:1:1, prepare a solution according to the ratio of adding 80g of fludarabine phosphate solid per liter of mixed solvent, heat to 45°C, stir until completely dissolved ;
[0035] (2) Distill under reduced pressure at 50°C. When the volume of the mixed solvent is reduced to 20%, stop the reduced-pressure distillation and cool down the mixed solvent to 1°C at a cooling rate of 2°C / min; let the crystal grow for 5 hours After obtaining the crystals, filter and dry in vacuum for 4 hours to obtain a white crystalline powder of fludarabine phosphate.
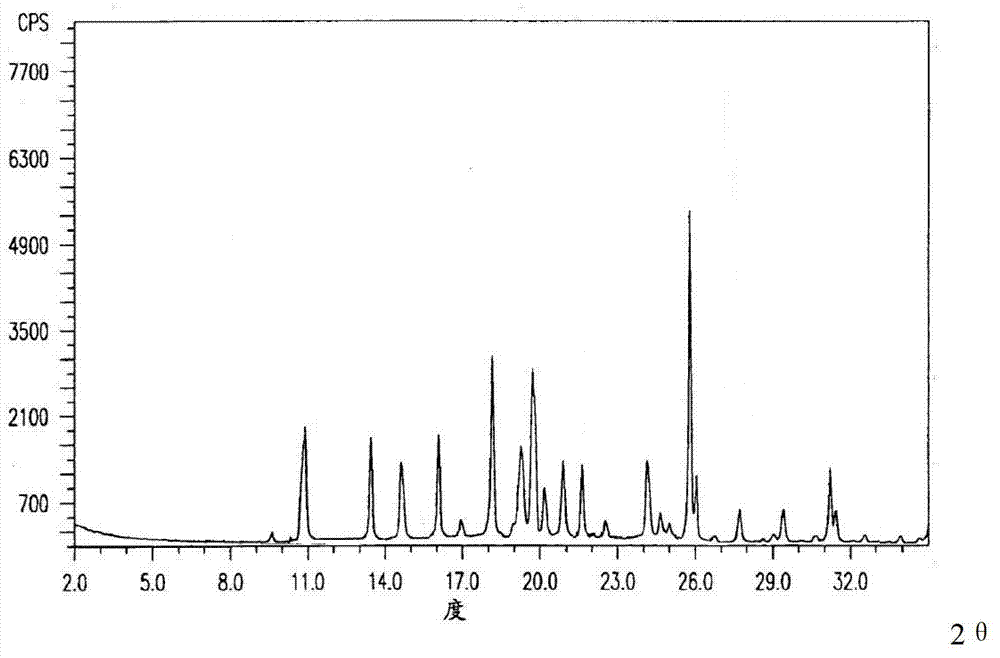
[0036] The prepared fludarabine phosphate is obtained by measuring the X-ray powder diffraction pattern of Cu-Kα ray as figure 1 Shown; detected by high performance liquid chromatography, its purity is 99.5%; melting point: 206 ~ 207 ℃.
Embodiment 2
[0038] (1) Prepare a mixed solvent according to the volume ratio of water: acetone and ethanol of 5:2:2, and prepare a solution according to the ratio of adding 100g of fludarabine phosphate solid per liter of mixed solvent, heat to 50°C, stir until completely dissolved ;
[0039] (2) Distill under reduced pressure at 50°C. When the volume of the mixed solvent is reduced to 10%, stop the reduced-pressure distillation, cool down the mixed solvent to 5°C at a cooling rate of 1.5°C / min, and let the crystal grow for 2 hours After obtaining the crystals, filter and dry in vacuum for 2 hours to obtain a white crystalline powder of fludarabine phosphate.
[0040] The prepared fludarabine phosphate is obtained by measuring the X-ray powder diffraction pattern of Cu-Kα ray as figure 1 Shown; detected by high performance liquid chromatography, its purity is 99.5%; melting point: 206 ~ 207 ℃.
Embodiment 3
[0042] (1) Prepare a mixed solvent according to the volume ratio of water: acetone and ethanol of 5:2:1, and prepare a solution according to the ratio of adding 50-120 g of fludarabine phosphate solid per liter of mixed solvent, heat to 45°C, and stir until completely Press to dissolve;
[0043] (2) Distill under reduced pressure at 45°C. When the volume of the mixed solvent decreases to 15%, stop the reduced-pressure distillation, and cool the mixed solvent to 2°C at a cooling rate of 1°C / min; stand for 4 hours to grow crystals After obtaining the crystals, filter and dry in vacuum for 3 hours to obtain a white crystalline powder of fludarabine phosphate.
[0044] The prepared fludarabine phosphate is obtained by measuring the X-ray powder diffraction pattern of Cu-Kα ray as figure 1 Shown; detected by high performance liquid chromatography, its purity is 99.5%; melting point: 206 ~ 207 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com