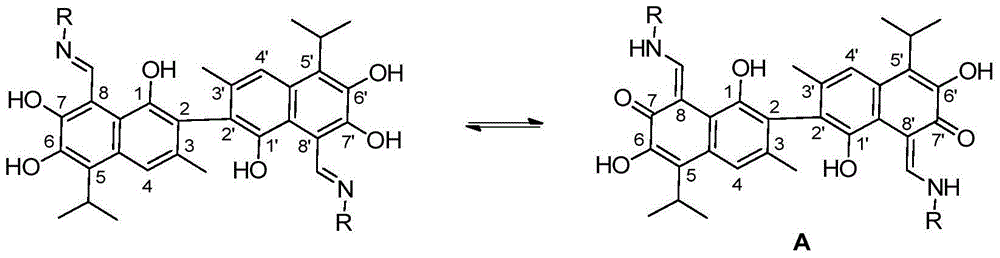
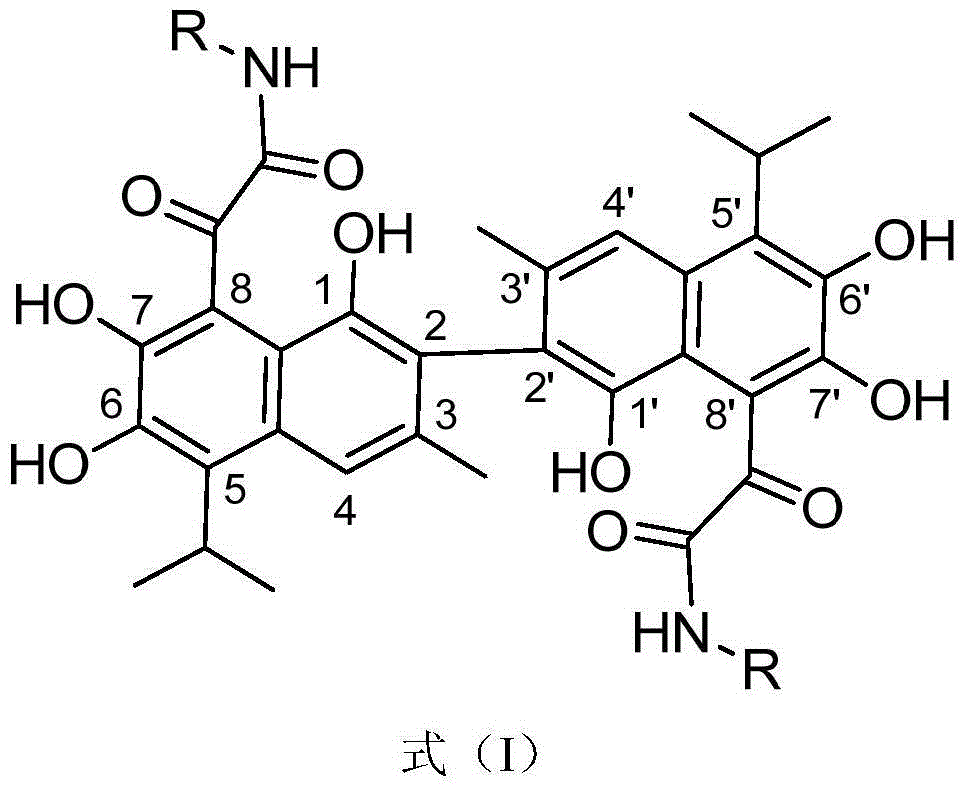
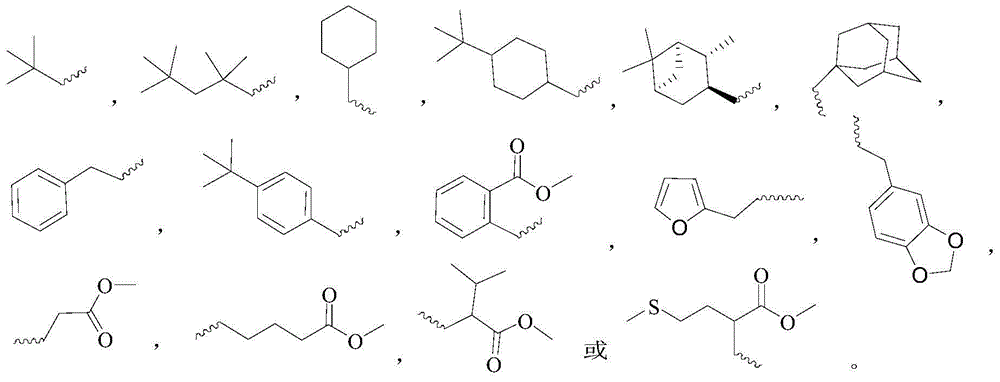
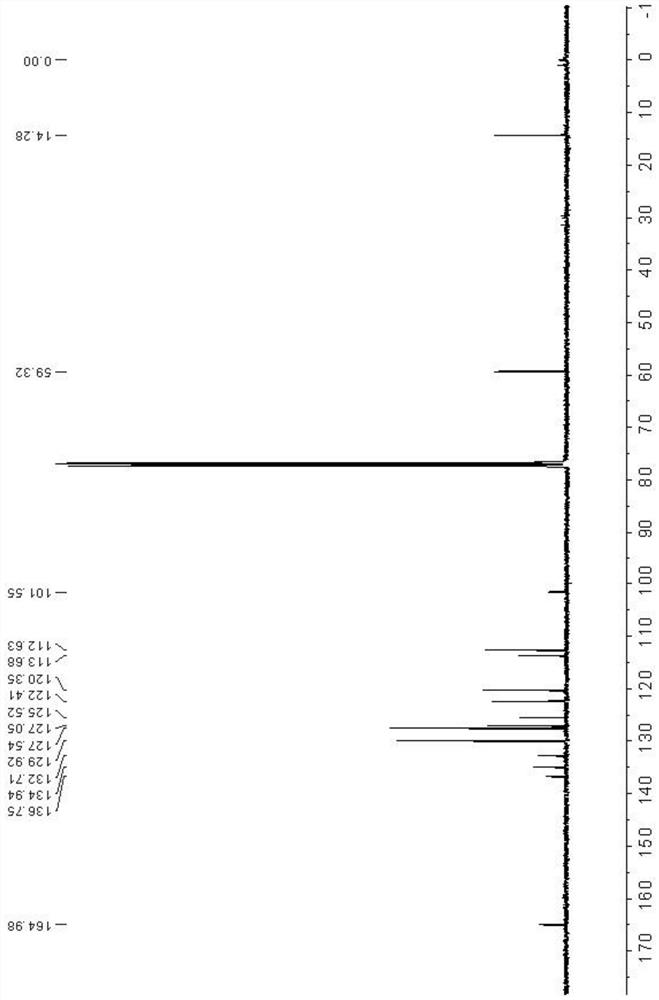
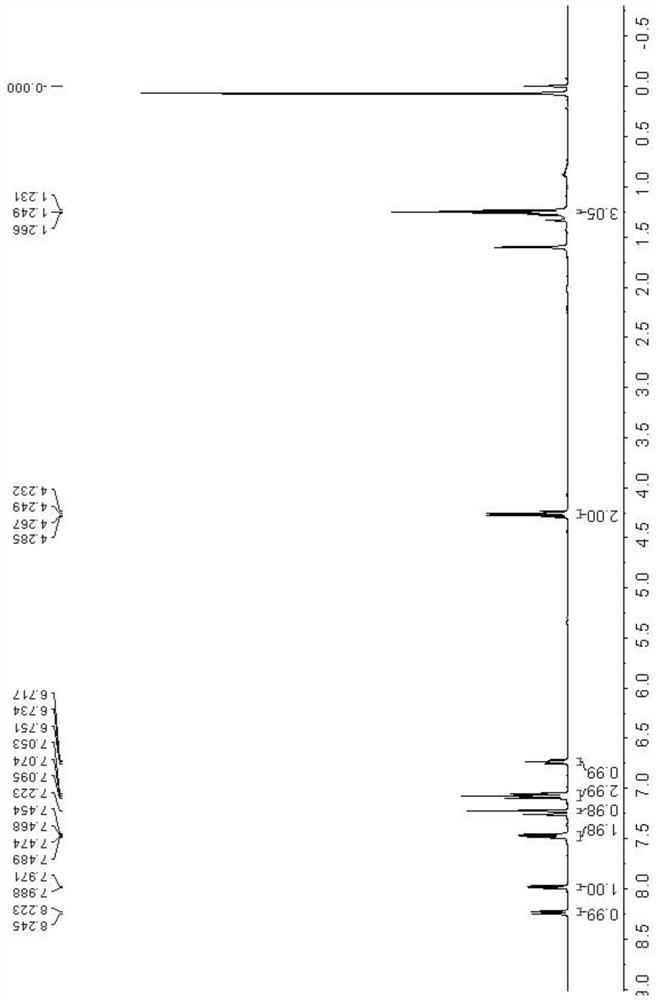
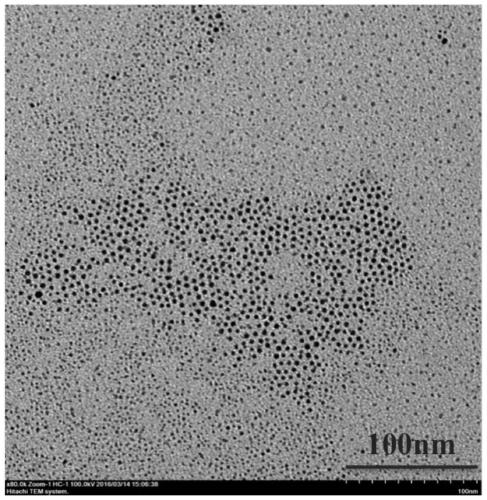
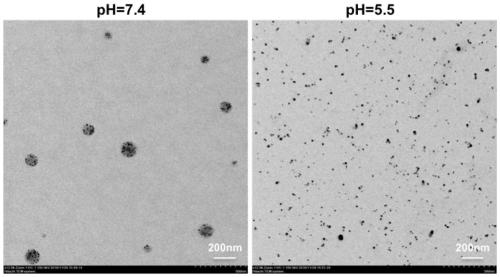
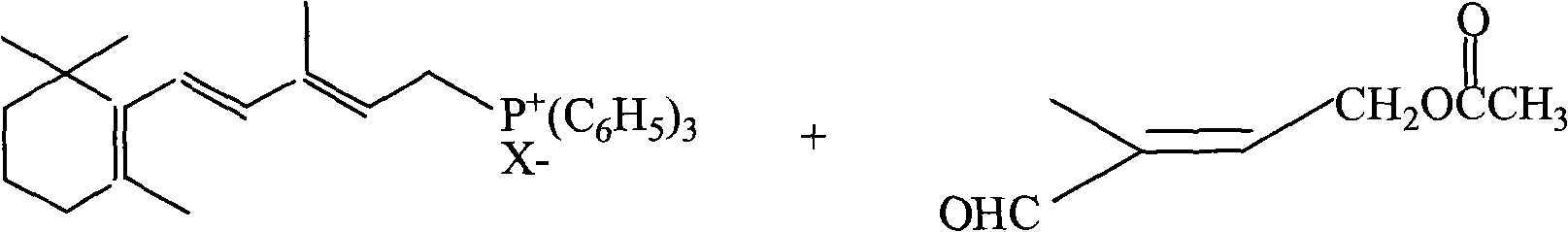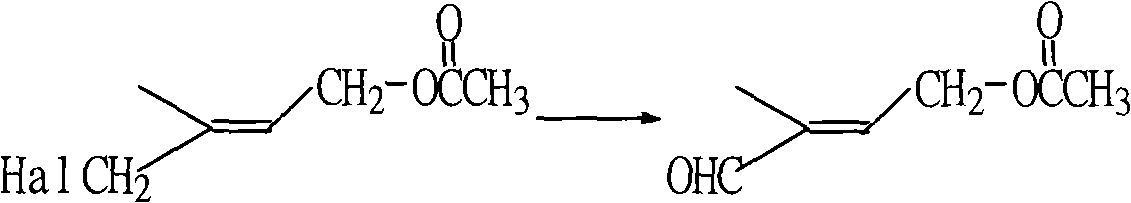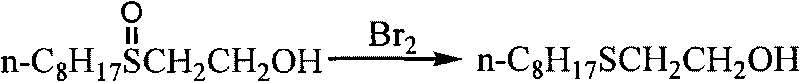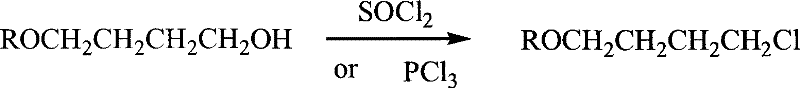Patents
Literature
50results about How to "Mild reaction system" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Crosslinked alginate-bacterial cellulose sponge and preparation method thereof
ActiveCN103536954AImprove mechanical propertiesSimple structureAbsorbent padsBandagesPolymer scienceFreeze-drying
The invention relates to the field of a biomedical material, and particularly discloses a crosslinked alginate-bacterial cellulose sponge and a preparation method thereof. The crosslinked alginate-bacterial cellulose sponge takes an alginate biomaterial and bacterial cellulose as raw materials. The crosslinked alginate-bacterial cellulose sponge is characterized in that a sponge product is prepared from the alginate biomaterial and the bacterial cellulose in manners of crosslinking and freeze-drying after being compounded, wherein the alginate biomaterial accounts for 80-95% of the sponge product in mass fraction; the bacterial cellulose accounts for 5-20% of the sponge product in mass fraction. The crosslinked alginate-bacterial cellulose sponge is abundant in used material sources, low in cost, controllable to process, and simple in production technology; the prepared crosslinked alginate-bacterial cellulose sponge has good mechanical property, liquid absorption property, water retention capacity and air permeability, and is applicable to bleeding healing of large seepage or a bleeding wound.
Owner:山东颐诺生物科技有限公司
Alginic acid-chitosan composite fiber for tranma repairing and preparation method thereof
InactiveCN103469365APromote healingImprove mechanical propertiesAbsorbent padsConjugated artificial filamentsFiberChemical reaction
The invention belongs to the field of biomedical materials, and particularly discloses an alginic acid-chitosan composite fiber for tranma repairing and a preparation method thereof. The alginic acid-chitosan composite fiber for the tranma repairing is characterized by comprising an alginic acid component of which the mass fraction is 50-95%, and a chitosan component of which the mass fraction is 5-50%. The processing technique disclosed by the invention is not involved with an organic reagent or an organic chemical reaction, so that the preparation method is mild in reaction system and controllable in condition; the residue problems of a toxic crosslinking agent or an assistant and the like do not exist; used materials, reagents and the like have good biosecurity; the security risk of clinical applications is greatly reduced. In addition, the technology disclosed by the invention is simple and controllable, and has the good product transformation feasibility; the difficulty of risk management in the preparation process is greatly reduced.
Owner:山东颐诺生物科技有限公司
Phenol wastewater treatment method and preparation method of CuO/ZSM-5 catalyst
ActiveCN105776494AReduce processing costsMild reaction systemMolecular sieve catalystsWater contaminantsEnvironmental chemistryPhenols
The invention discloses a phenol wastewater treatment method and a preparation method of a CuO / ZSM-5 catalyst. Compared with the prior art, CuO is loaded on ZSM by an impregnation method to prepare a composite catalyst with good performance; persulfate is catalyzed to generate sulfate radicals to play stronger oxidizing ability; the treatment cost is reduced, the requirements on reaction equipment are not high, and the method is simple and easy to implement, has high operability and facilitates practical promotion and application; and moreover, the mineralization conversion of pollutants is thorough, the pollutants are converted into corresponding nontoxic substances, secondary pollution is avoided, the highest conversion rate of phenol can reach 99%, and the method has remarkable environmental advantages and economic advantages.
Owner:合肥沃雨环保科技有限公司
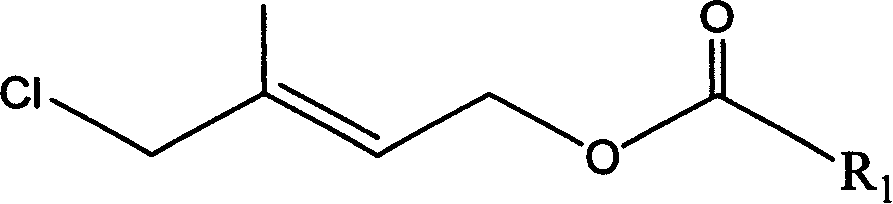
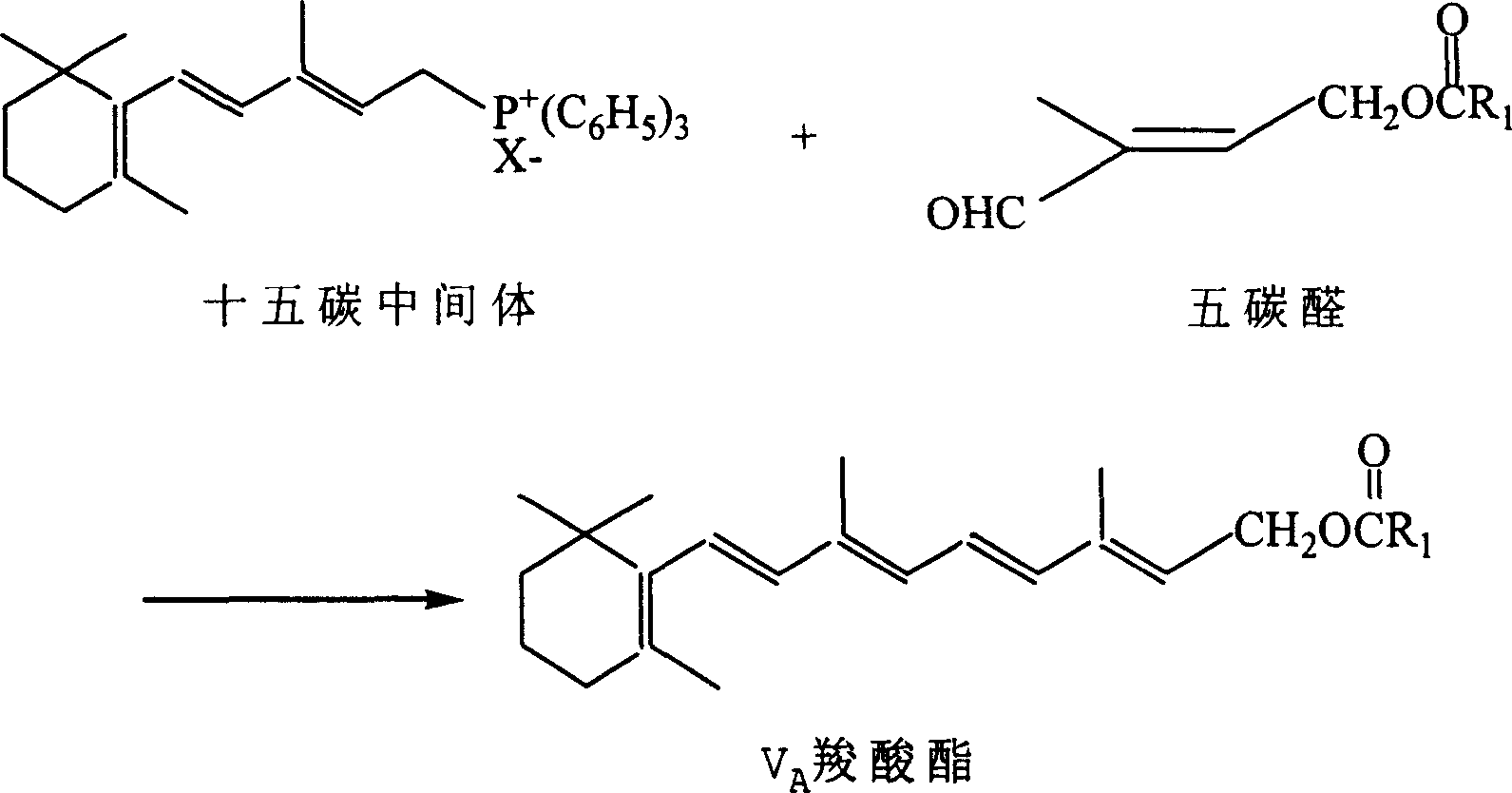
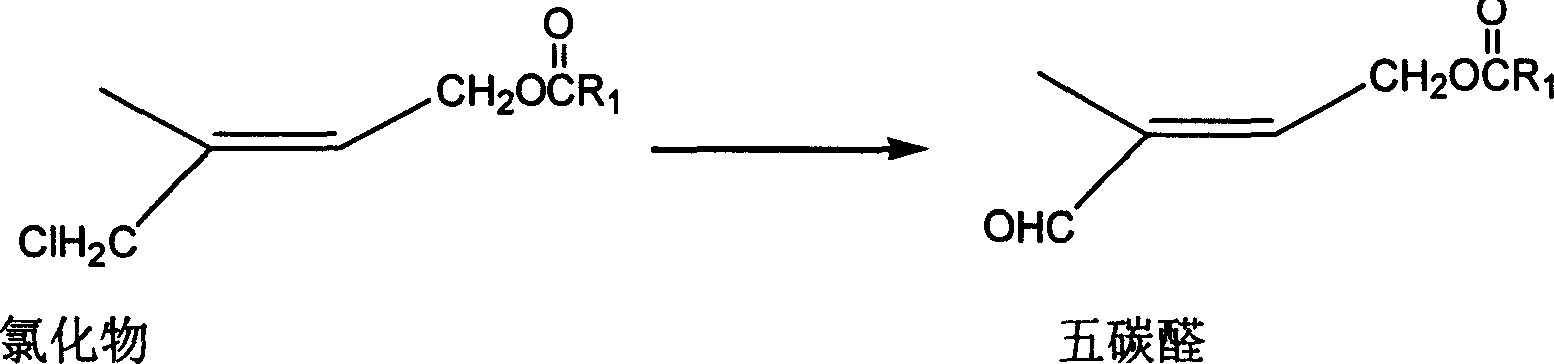
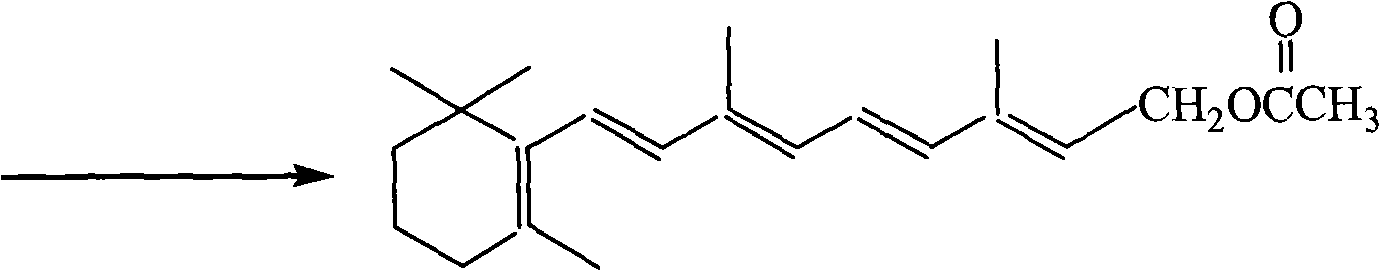
Method for preparing 1-chloro-2-methyl-4-alkylacyloxy-2-butene
ActiveCN1844077AHigh content of available chlorineLess hetero ionsPreparation from carboxylic acid halidesOrganic compound preparationPolymer science2-Butene
The method provides a Method for preparation of vitaminA derivant's important intermediate- 1-chloro-2-methyl-4-hydrocarbon acyloxy-2-butylene, this invention adopting isoprene as material to carry through chlorohydrination reaction; the getted product has high content, high produce rate, convenierce operate, little waste and easy to commercial manufacture.
Owner:SHAOXING UNIVERSITY +1
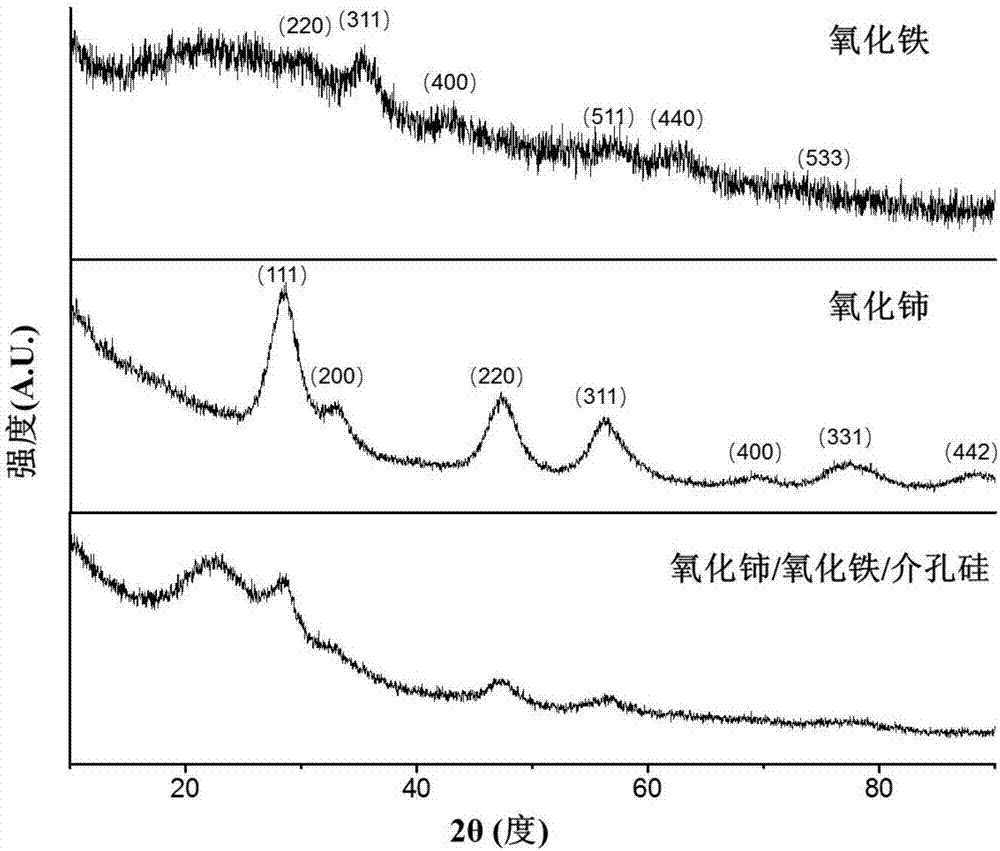
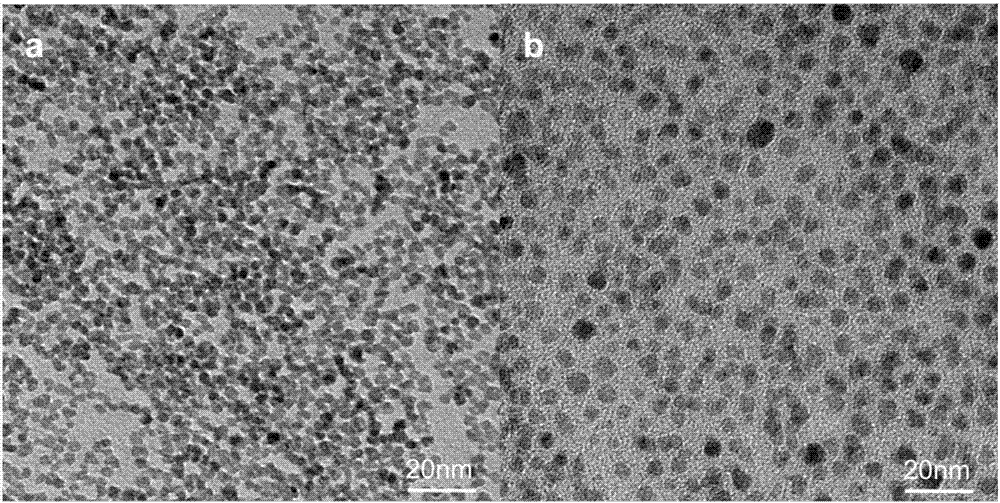
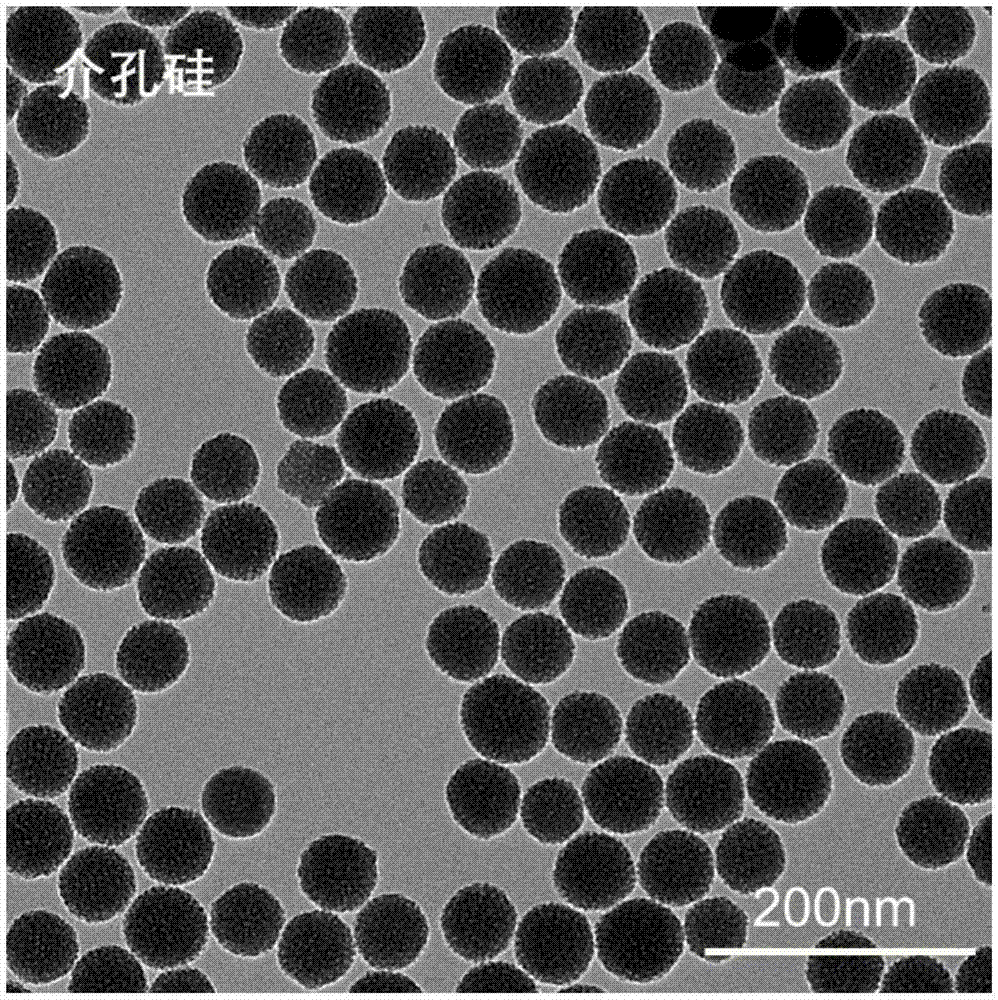
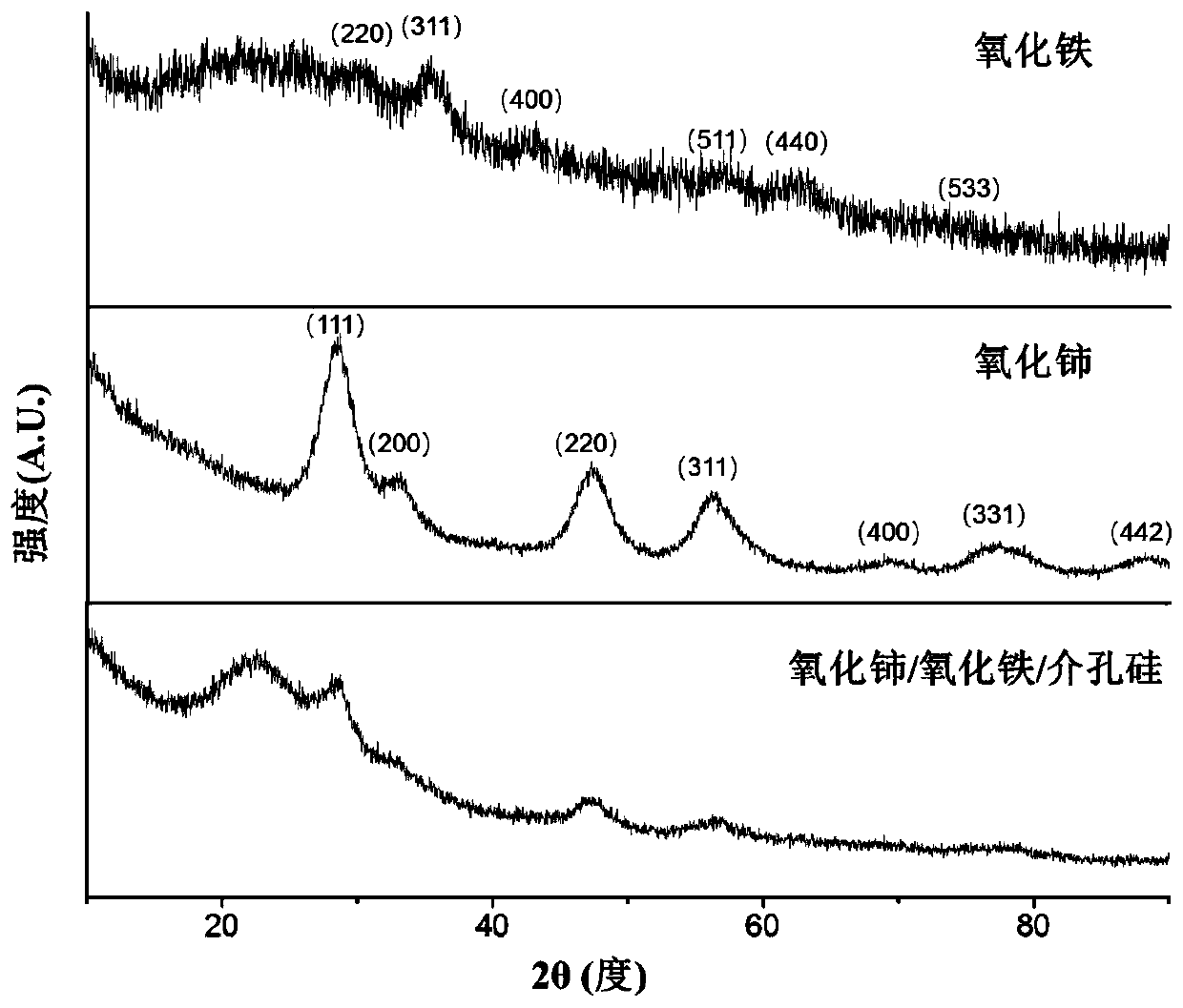
Cerium oxide/iron oxide/mesoporous silica nano composite material as well as preparation method and application thereof
ActiveCN106860427AGood biocompatibilityGood potential for clinical translationNervous disorderInorganic non-active ingredientsNMR - Nuclear magnetic resonanceMagnetic resonance imaging contrast medium
The invention relates to a cerium oxide / iron oxide / mesoporous silica nano composite material. The surfaces of amino-modified mesoporous silica nanoparticles are respectively modified by ligand-converted cerium oxide nanocrystals and ligand-converted iron oxide nanocrystals. The invention also relates to a preparation method and application of the cerium oxide / iron oxide / mesoporous silica nano composite material. Not only can the composite material carry a drug for treating Alzheimer apostrophe s disease (AD), but also can remove reactive oxide species (ROS) in AD-similar cells, and can be used for diagnosis of the AD as a nuclear magnetic resonance imaging contrast agent, thus realizing integration of diagnosis and treatment of the AD.
Owner:ZHEJIANG UNIV
Preparing method of cellulose composite sponge
InactiveCN107189111AFast water absorptionAvoid defects such as insufficient absorption capacityCelluloseCross-link
The invention discloses a preparing method of cellulose composite sponge. The preparing method of the cellulose composite sponge comprises the following steps of 1, preparing an aqueous solution of HPMC and an aqueous solution of CMC separately, and conducting even stirring to make the aqueous solution of HPMC and the aqueous solution of CMC fully dissolved; 2, proportionally mixing the aqueous solution of HPMC and the aqueous solution of CMC which are prepared in the step 1, and conducting even mixing; 3, freezing and drying the evenly mixed liquid obtained in the step 2; 4, cross-liking the frozen and dried mixture obtained in the step 3 in a cross-linking agent; 5, using a refrigerator to freeze and dry the cross-linking product cross-linked in the cross-linking agent obtained in the step 4 to obtain a spongy product; 6, packaging and sterilizing the spongy product obtained in the step 5 to obtain the cellulose composite sponge used for hemostasis nursing. According to the preparing method of the cellulose composite sponge, the water absorption and the water binding capacity of the composite material are improved, and the wet strength and flexibility are remarkably improved.
Owner:浙江美华鼎昌医药科技有限公司
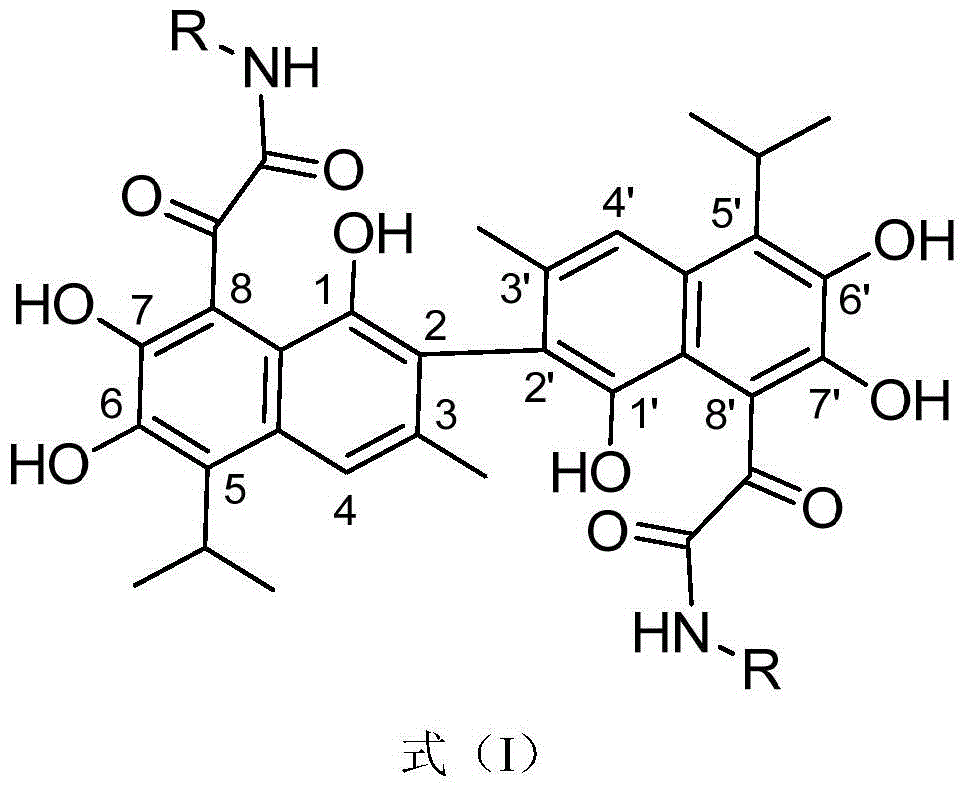
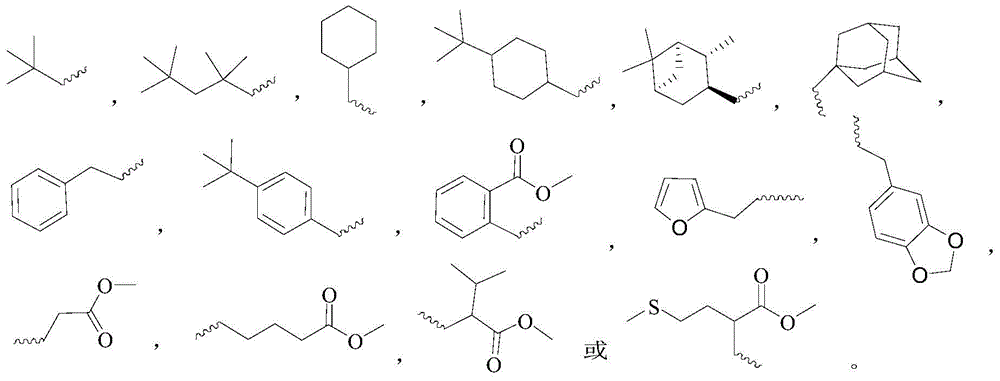
Novel gossypol derivatives, and preparation method and antineoplastic application thereof
ActiveCN104817472ARetain active phenolic hydroxylImprove biological activityOrganic compound preparationCarboxylic acid amides preparationOrganic synthesisSolvent
The invention specifically discloses novel gossypol derivatives, and a preparation method and application thereof, belonging to the technical field of organic synthesis. According to the method, gossypol and isonitrile are used as raw materials and undergo a stirring reaction in a polar solvent at 70 to 80 DEG C for 24 and 36 h, then simple filtering and separating are carried out, and thus, a series of the gossypol derivative with modified aldehyde groups are prepared through one-step reaction. The preparation method and post-treatment are simple; a reaction system is mild; and the synthesized novel gossypol derivatives retain all the active phenolic hydroxyl groups unique to gossypol to a greatest extent, and it is estimated that all the excellent biological activities of gossypol are retatined and the problem of toxic aldehyde groups carried by gossypol is overcome. Most of the gossypol derivatives prepared in the invention have good anti-prostatic cancer activity and can be applied in the antineoplastic field.
Owner:江苏度未生物工程科技有限公司
NIR-II conjugated nano particles, and preparation method and application thereof
ActiveCN109529034AMild reaction systemControllable conditionsEnergy modified materialsEchographic/ultrasound-imaging preparationsChemistryMolecular self-assembly
The invention relates to NIR-II conjugated nano particles and a preparation method and application thereof. The NIR-II conjugated nano particles comprise NIR-II conjugated small molecules and amphiphilic polymers; a structural formula of the NIR-II conjugated small molecules is as follows; and the amphiphilic polymers are self-assembled and encapsulated on the NIR-II conjugated small molecules. The nanoparticles have good biocompatibility, high NIR-II photothermal conversion efficiency, good photoacoustic imaging effect and NIR-II tumor photothermal therapy effect.
Owner:ZHEJIANG UNIV
GTR (guided tissue regeneration) membrane and preparation method thereof
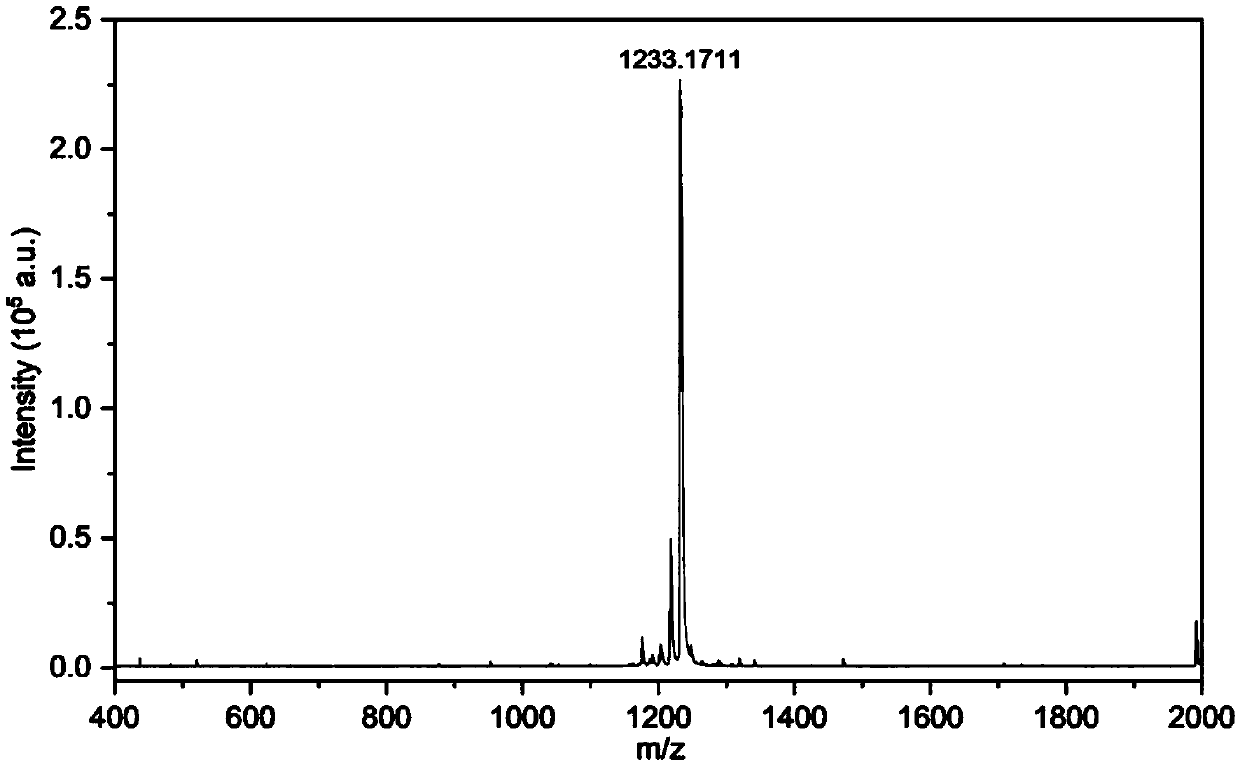
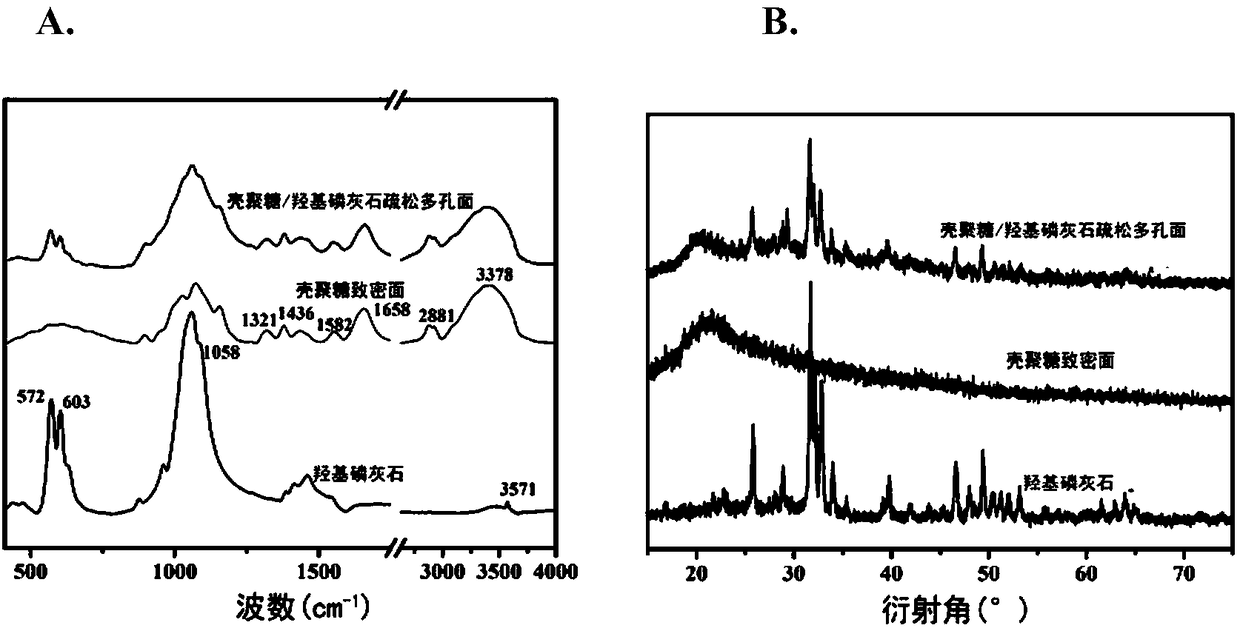
The invention discloses a GTR (guided tissue regeneration) membrane and a preparation method thereof. The GTR membrane comprises a compact layer with a chitosan matrix and a loose porous layer with achitosan and calcium phosphate compound matrix. A three-electrode system is adopted, compacted chitosan hydrogel is firstly deposited near cathodes, then, loose chitosan and calcium phosphate hydrogelis deposited on the chitosan hydrogel, double-layer hydrogel is obtained, and the GTA membrane is obtained after drying. The loose porous layer of the GTA membrane has osteogenic activity and can provide more structural space for cell growth and good microenvironment for proliferation and differentiation and promote fusion of bone cells, and meanwhile, the compact layer can shield fibrous tissue.The preparation method is simple, rapid, green in process and controllable for membrane structure.
Owner:江苏博创生物科技有限公司
GPTR (Guided Periodontal Tissue Regeneration) barrier membrane, and preparation method and application thereof
ActiveCN106310366AEasy to operateMild reaction systemTissue regenerationProsthesisTissue repairFreeze-drying
The invention discloses a GPTR (Guided Periodontal Tissue Regeneration) barrier membrane, and a preparation method and application thereof, and belongs to the field of biomedical materials. The GPTR barrier membrane is a tissue regeneration barrier membrane which is of a double-layer asymmetric structure with a compact layer and a porous layer, and can be used for tissue repairing. Preparation of the GPTR barrier membrane comprises the following steps: carrying out tape casting on a soybean protein / chitosan complex solution, thus preparing the compact layer of a double-layer asymmetric barrier membrane; carrying out freeze drying on the compact layer, thus preparing the porous layer of a double-layer asymmetric composite barrier membrane; enabling the double-layer asymmetric composite barrier membrane to carry bioactive factors, thus obtaining the tissue regeneration barrier membrane. According to the GPTR barrier membrane and the preparation method, disclosed by the invention, the operation steps are simple, a preparation process of the tissue regeneration barrier membrane has no addition of toxic reagents of aldehydes and the like, a reaction system is moderate, and the bioactivity of two natural high polymer materials and the added bioactive factors can be better reserved.
Owner:WUHAN INST OF BIOENG
Preparation method of asymmetric Gemini fluorocarbon surfactant
InactiveCN111514811AEasily biodegradableLow reaction temperatureOrganic compound preparationTransportation and packagingActive agentSurface-active agents
The invention discloses a preparation method of an asymmetric Gemini fluorocarbon surfactant. The preparation method comprises the following steps: step 1, mixing succinyl chloride and a solvent; step2, mixing triethylamine and 1H, 1H-perfluorohexylamine, and drwowise adding the mixed solution at a constant speed to conduct a reaction; step 3, mixing triethylamine and hydrocarbon long-chain primary amine, dropwise adding the mixed solution at a constant speed, and carrying out a reaction; and step 4, continuously dropwise adding 10-20 g of ethanol into a three-neck round-bottom flask, and cooling to room temperature after dropwise adding is completed. The asymmetric Gemini fluorocarbon surfactant is superior to or equivalent to a conventional fluorocarbon surfactant on the market in surface tension reducing capacity and efficiency; meanwhile, the synthesized fluorocarbon surfactant molecule contains a hydrocarbon long chain with a certain length, and has a remarkable effect of reducing the interfacial tension of oil and water.
Owner:SHAANXI YUTENG IND
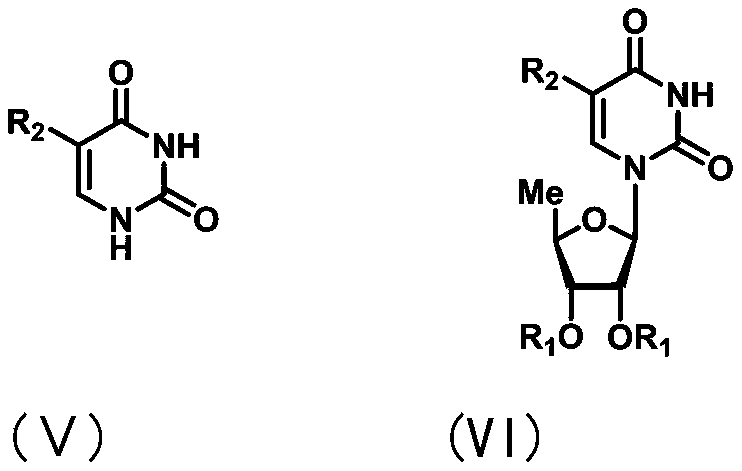
Uracil nucleoside derivative and method for preparing doxifluridine medicine by using uracil nucleoside derivative
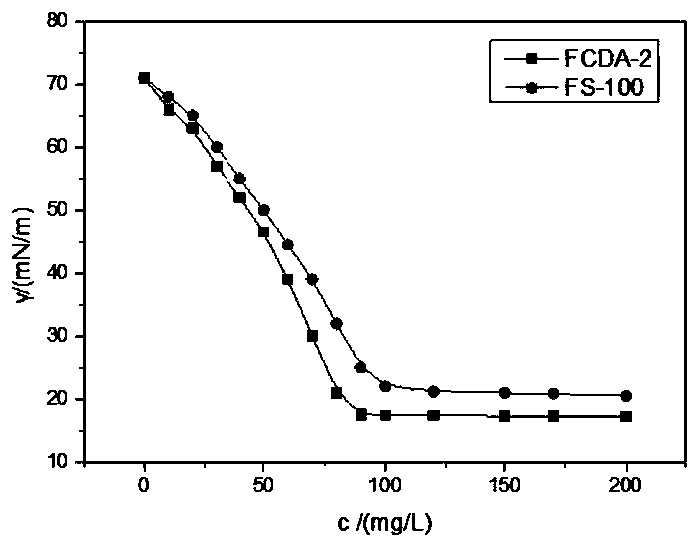
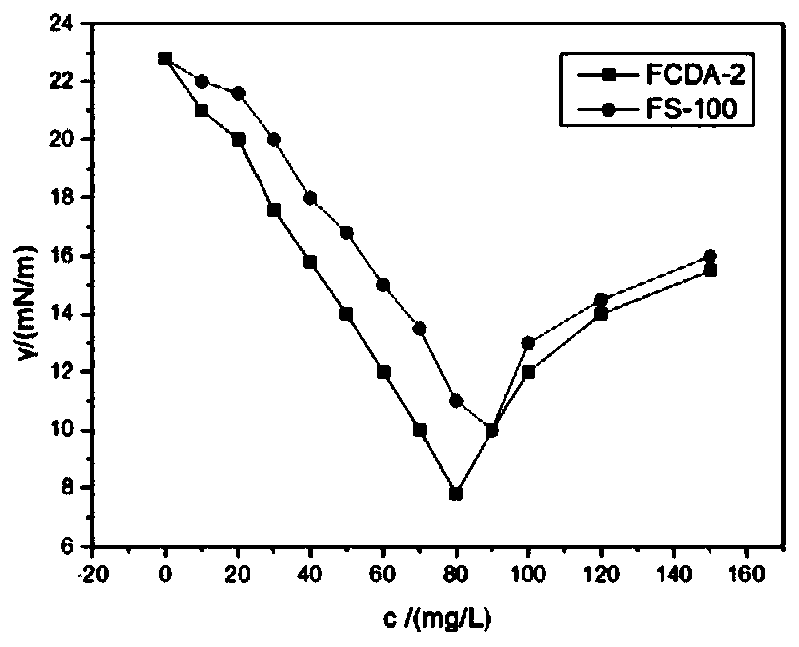
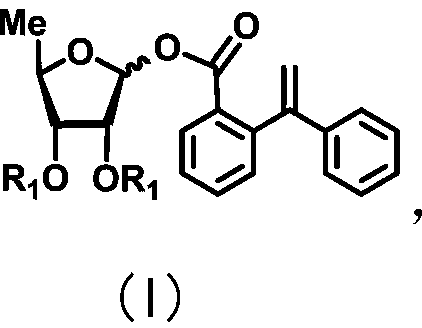
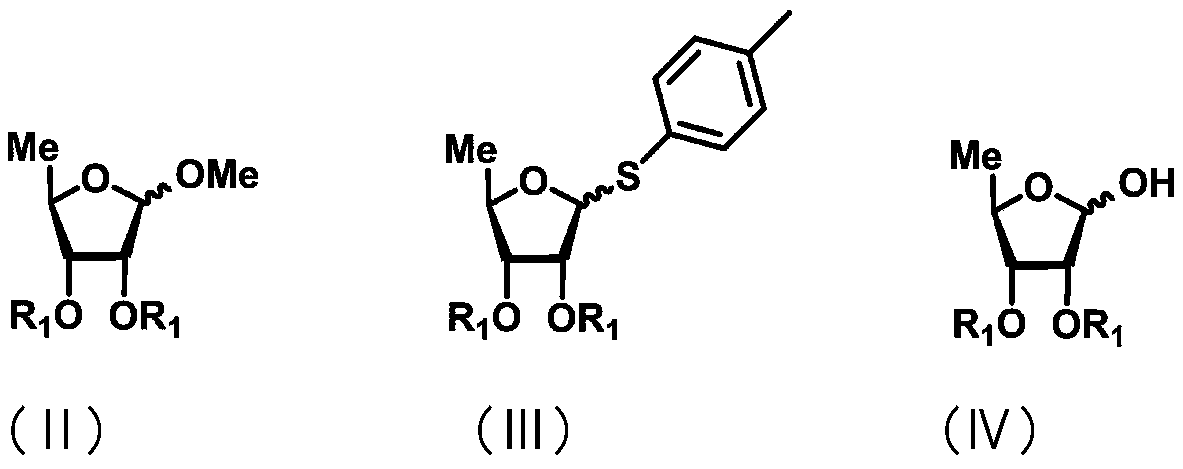
ActiveCN111072734AMild reaction systemEfficient responseSugar derivativesSugar derivatives preparationBenzoic acidFormic Acid Esters
The invention discloses a 5-deoxy-D-ribofuranose 1-[2-(1-styryl) benzoate] derivative as shown in a general formula (I) and a preparation method of the derivative, wherein the structure of the generalformula (I) is as follows, and the invention discloses a method for preparing a uracil nucleoside derivative and an antitumor drug doxifluridine by taking the derivative as shown in the general formula (I) as a raw material. The 5-deoxy-D-ribofuranose 1-[2-(1-styryl) benzoate] derivative used as a reaction raw material can be activated as a glycosyl donor under the condition of a catalytic amountof lewis acid trimethylsilyl trifluoromethanesulfonate and N-iodosuccinimide. The method avoids traditional use of equivalent or excessive lewis acid, has a mild reaction system, has no other side reaction, has efficient reaction, and has a yield up to 98%.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Injectable alginate-based biomaterial for adjuvant therapy of heart failure and preparation method thereof
The invention relates to an injectable type alginic acid radical biological material for heart failure adjuvant therapy and a preparation method thereof. The preparation method comprises steps of: preparing two systems of a sodium alginate system and a cross-linking agent system, mixing the two components uniformly by utilizing a three-way syringe and preparing the injectable type alginic acid radical biological material for heart failure / myocardial infarction adjuvant therapy after a proper time of balancing. The materials and processing technologies which are adopted by the invention do not involve organic reagents or organic chemical reactions, the reaction systems are moderate, the conditions are controllable, and the problem of residues of toxic cross-linking agents or auxiliaries cannot be caused; a hydrogel which is prepared according to the technical scheme is injected into the spherical expanding myocardium after heart failure by using the syringe No.271 / 2, so that cardiac ventricles can be helped to reshape, the sizes of the cardiac ventricles can be effectively reduced, and the tension of ventricle walls is decreased; and the biological material can be used for heart failure adjuvant therapy, has good mechanical properties and biological properties as well as good cytocompatibility and is convenient and reliable to operate.
Owner:奚廷斐
Process for synthesizing 1-chlorine-2-methyl-4-acetoxy-2-butylene
ActiveCN101270048AHigh content of available chlorineLess hetero ionsOrganic compound preparationCarboxylic acid esters preparationButeneAcetic anhydride
The present invention discloses a synthetic method of 1-chlorine-2-methyl-4-acetoxy-2-butene, an important intermediate for synthesizing V acetate. The existing method adopts chlorohydrin reaction to prepare the product and has the problems of poor yield rate and content. In the method, isoprene is used as the raw material for the chlorohydrin reaction so as to prepare the mixture of addition products of 1-chlorine-2-hydroxy-2-methyl-3-butene and 1-chlorine-2-methyl-4-hydroxy-2-butene. Under the influence of acid catalysis, the mixture reacts with acetic anhydride to prepare the 1-chlorine-2-methyl-4-acetoxy-2-butene. The method is characterized in that the chlorohydrin reaction is done in the reaction system that consists of tetrachloroglycine urea and water. The raw materials used in the method has high content of available chlorine and less heteroion; the reaction system is mild; the content and the yield of the product are high; the three-waste is greatly reduced; the cost is low; and the method has high industrial value.
Owner:ZHEJIANG MEDICINE CO LTD +1
Method for synthesizing 2-ethoxy n-octyl thioether through three phase transfer catalysis without solvent
InactiveCN101698655AReduce manufacturing costSimple reaction systemSulfide preparationChemistryFine chemical
The invention discloses a new method for synthesizing 2-ethoxy n-octyl thioether through three phase transfer and catalysis without solvent, belonging to the technical field of fine chemicals synthetization. The method takes octyl mercaptan and 2-chlorohydrin as main raw materials, and uses organic macromolecule materials as the carrier, surface chemical modification is carried out, grafted polyethyleneglycol and quaternary ammonium salt with proper link and group structures are taken as the three phase transfer catalyst, the reaction is carried out for 6-12 hours at normal pressure and temperature of 40-90 DEG C without solvent to synthesize 2-ethoxy n-octyl thioether. Compared with the existing methods, the invention is characterized by available raw materials, low cost of production, mild reaction conditions, easy separation of the product after reaction, energy saving and consumption reducing. The three phase transfer catalyst has high activity, excellent selectivity, and good mechanical strength, and is easy to recycle from the reaction system. The invention has better industrialization application prospect.
Owner:ZHENGZHOU UNIV
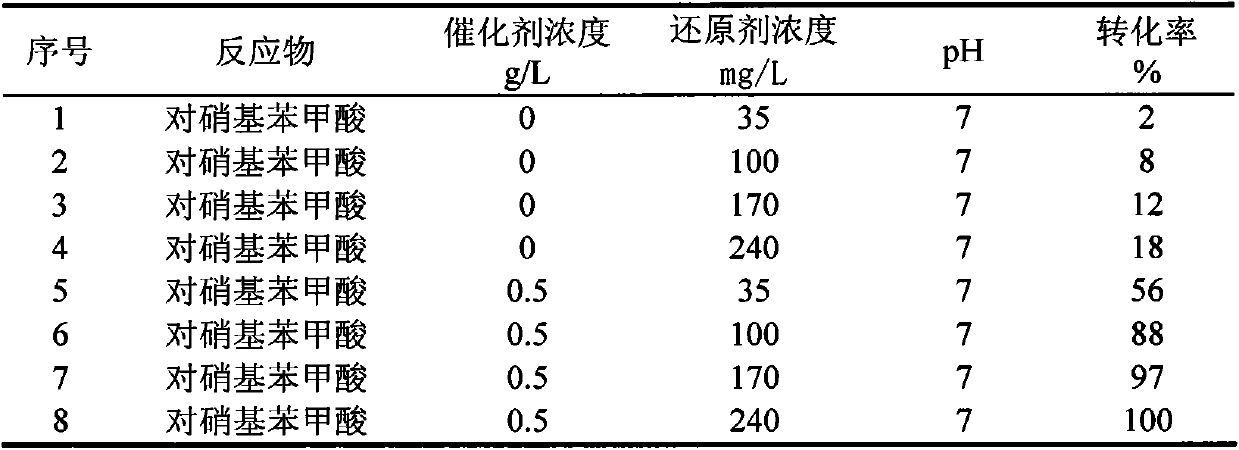
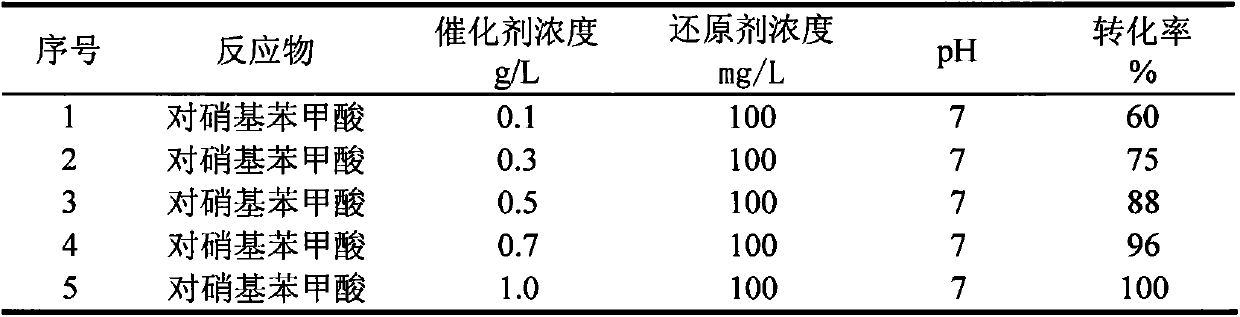
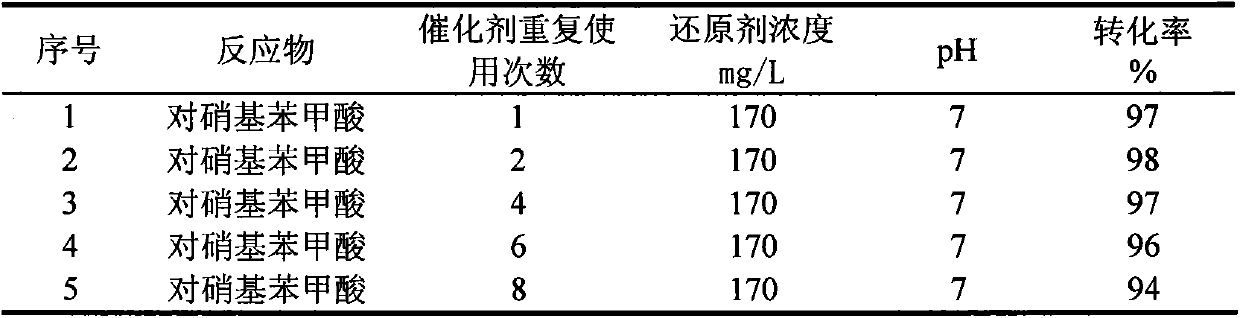
Method for treating p-nitrobenzoic acid wastewater with activated carbon through catalytic reduction
InactiveCN104045141AMild reaction systemResponse equipment requirements are lowWater contaminantsWater/sewage treatment by reductionP-nitrobenzoic acidNitrobenzoic acid
The invention belongs to the technical field of wastewater treatment for environmental protection and relates to a method for treating p-nitrobenzoic acid wastewater with activated carbon through catalytic reduction. The method comprises the following main steps: adding a buffer solution and a certain concentration of p-nitrobenzoic acid wastewater to an anaerobic simulation reactor containing activated carbon weighed in advance, filling 99.99% of high-purity nitrogen into a reaction system (so as to remove oxygen in the system to form an anaerobic environment), adding a certain concentration of reducing agent, namely a sodium thiosulfate solution, to the system and finally putting the reactor in a rotary oscillator and carrying out oscillating reaction at normal temperature and pressure. The method has the advantages that the reaction system is mild, the reaction is quick and efficient and the treatment cost is low, so that the method can conduce to solving practical problems and has obvious economic and environmental advantages.
Owner:BEIJING NORMAL UNIVERSITY
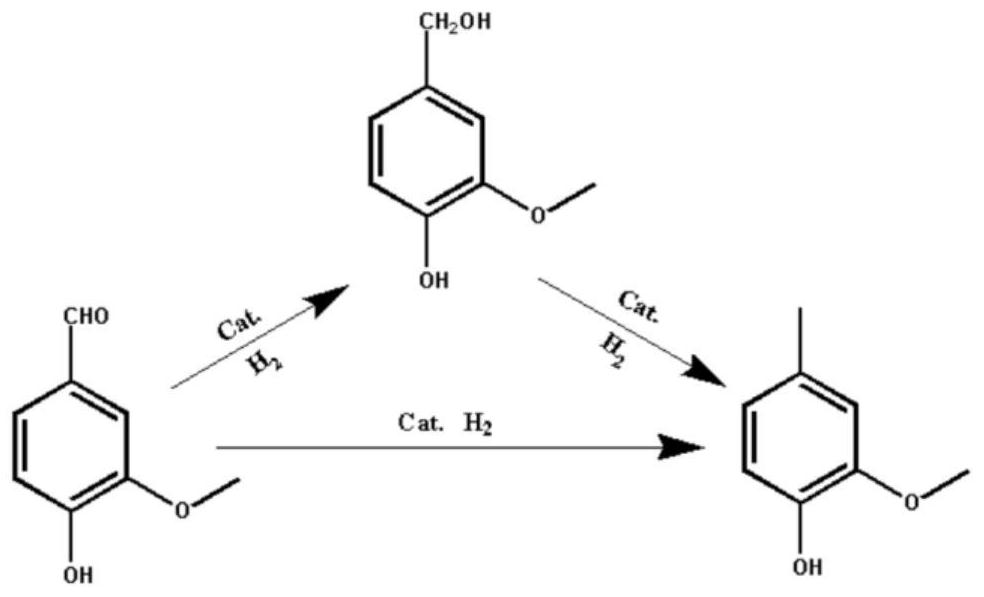
Preparation method of 2-methoxy-4-methylphenol based on selective hydrodeoxygenation of vanillin
PendingCN114573429APore size is easy to adjustImprove conductivityOrganic chemistryOrganic compound preparationIridiumPtru catalyst
The invention discloses a 2-methoxy-4-methylphenol preparation method based on vanillin selective hydrodeoxygenation, which comprises the following steps: adding a reactant vanillin into a reaction kettle containing a certain amount of solvent, and carrying out hydrodeoxygenation reaction in the presence of a catalyst to obtain 2-methoxy-4-methylphenol, the catalyst is a porous carbon nanosphere loaded with a metal active component, the expression of the catalyst is M (at) CNS, M is any one of metal ruthenium, platinum, palladium, iridium, rhodium and gold, and CNS is a porous carbon nanosphere carrier. The porous carbon nanosphere not only has the characteristics of large specific surface area, abundant shell pore channels and easy regulation and control of pore diameter, but also has high dispersity and uniform size of loaded metal nanoparticles. The catalyst can realize high-efficiency and high-selectivity hydrodeoxygenation of vanillin under mild conditions to prepare 2-methoxy-4-methylphenol.
Owner:SHAANXI UNIV OF SCI & TECH
Alginic acid-hyaluronic acid in situ tissue engineering cell scaffold and its preparation method
InactiveCN103550830AImprove mechanical propertiesImprove fill/support performanceProsthesisPerylene derivativesAlginic acid
The invention provides an alginic acid-hyaluronic acid in situ tissue engineering cell scaffold and its preparation method. The preparation method comprises the following steps: alginic acid or its derivative and hyaluronic acid or its derivative are respectively made into solutions by the use of normal saline or physiological buffer; any one of the above solutions is mixed with cells which need to be loaded and then is mixed with the other remaining solution; and balancing lasts for a period of time at room temperature so as to obtain the alginic acid-hyaluronic acid in situ tissue engineering cell scaffold. The prepared alginic acid-hyaluronic acid in situ tissue engineering cell scaffold effectively prolongs degradation time of alginic acid and hyaluronic acid in vivo. By appropriate intramolecular crosslinking, mechanical properties and tissue filling / supporting performance of the scaffold are raised greatly. The scaffold has good security and validity. The preparation method of the alginic acid-hyaluronic acid in situ tissue engineering cell scaffold does not involve any organic reagent; the reaction system is simple and mild; and clinic application security is good.
Owner:PEKING UNIV
Ion specific filter membrane/mesoporous silicon composite material, nano sensor, product and application thereof
ActiveCN109876157AHigh selectivityHigh sensitivityIn-vivo testing preparationsExtracellularNanoparticle
The invention relates to an ion specific filter membrane / mesoporous silicon composite material, a nano sensor, a product and an application thereof. The ion specific filter membrane / mesoporous siliconcomposite material includes mesoporous silica nanoparticles and an ion specific filter membrane deposited on the surface of the mesoporous silica nanoparticles. The nano sensor comprises the ion specific filter membrane / mesoporous silicon composite material and a corresponding ion indicator adsorbed within the mesoporous silica nanoparticles. The nano sensor can perform dynamical imaging for changes of free-moving in-vivo extracellular ion concentration with high selectivity and high sensitivity, and reflects the brain nerve activity.
Owner:ZHEJIANG UNIV +2
Acid-basebifunctional solid catalyst as well as preparation method and application thereof
InactiveCN110227429AReduce generationAchieving acid-base synergistic catalysisOrganic chemistryMolecular sieve catalystsBifunctionalSolvent
The invention discloses an acid-basebifunctional solid catalyst as well as a preparation method and application thereof. The preparation method of the acid-base bifunctional solid catalyst comprises the following steps: mixing a templating agent and absolute ethanol, adding a Lewis acid, performing mixing, adjusting the pH to 1-5, and adding an acid to prepare a mixed solution; performing ultrasonic treatment on the above mixed solution, and performing solvent volatilization self assembly to obtain residues after volatilization self assembly; and performing calcination on the above residues toobtain the acid-base bifunctional solid catalyst. According to the preparation method of the acid-base bifunctional solid catalyst provided by the invention, and the solid catalyst prepared by the method has acid and alkali catalytic active sites, realizes acid-base synergistic catalysis in the solid catalyst, accelerates the progress of a catalytic reaction, reduces generation of by-products, and has a sugar conversion rate of 99.3% and a 5-hydroxymethylfurfural yield of 80.2% when used to catalyze preparation of the 5-hydroxymethylfurfural from biomass sugar.
Owner:GUANGDONG UNIV OF TECH
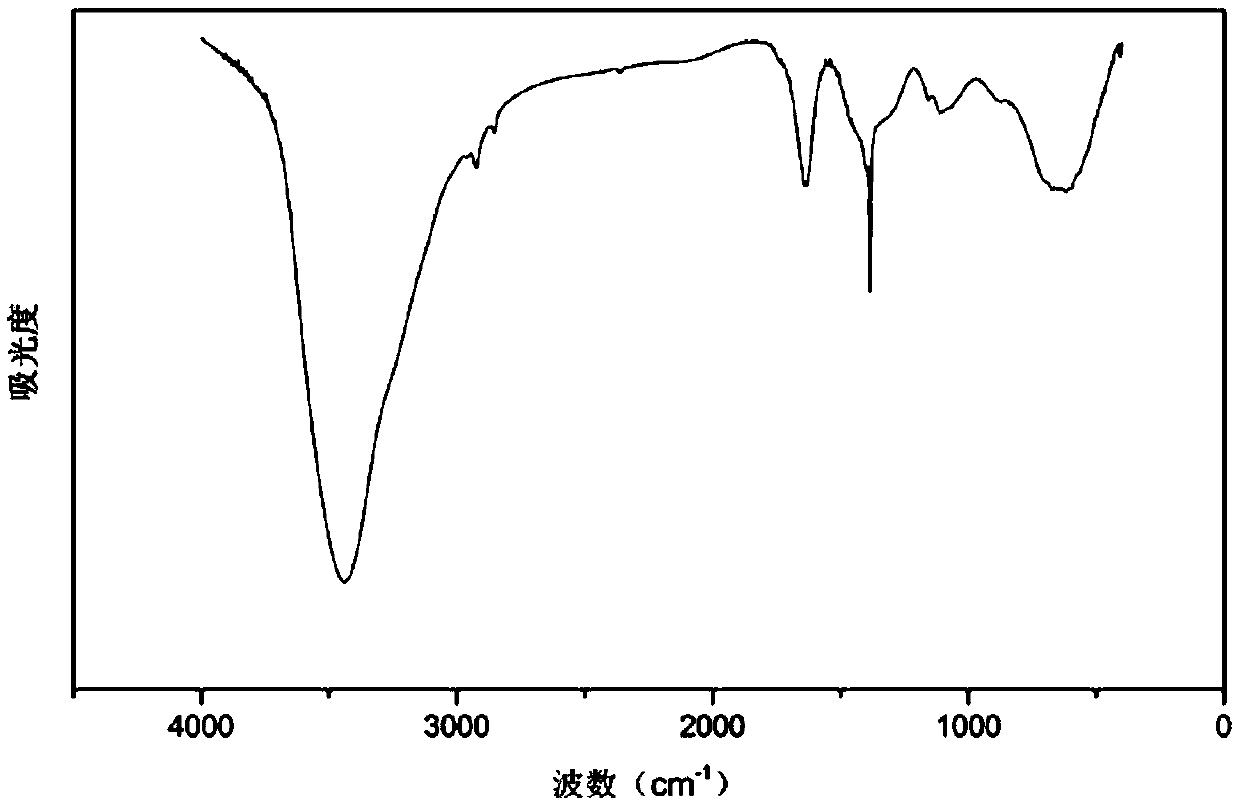
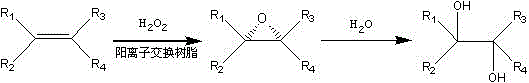
Method for preparing 4-alkoxy-1-chlorobutane
InactiveCN101177384BReduce usageMild reaction systemOrganic chemistryOrganic compound preparationPressure reductionAlkaline catalyst
The invention discloses a preparation method for 4-alkoxy-1-chlorobutane used as a synthetic resin raw material and a pharmaceutical intermediate compound. The prior method adopting basic catalyst has shortcomings that: the basic catalyst is difficult to be separated from the reaction system when the reaction is completed, and is difficult to be cleaned even if a large amount of water is used, thereby which brings difficulties to the fine purification for the product. The invention adopts 4-alkoxy-1-chlorobutane as the raw material, which is reacted with thionyl chloride under the existence of quaternary ammonium salt catalyst so as to get the product 4-alkoxy-1-chlorobutane, and then the product is implemented with water scrubbing and stratification for removing the catalyst and destroying the superfluous thionyl chloride, and then the product is washed with lye and water, and the pure product is obtained after distilled in atmospheric pressure and pressure reduction finally. The invention has the advantages of less secondary reaction, convenient post treatment since the product is implemented with water scrubbing and stratification for removing the catalyst and destroying the superfluous thionyl chloride, good catalytic result of the catalyst, less use amount of thionyl chloride, and high content and yield of the product.
Owner:GAOYOU CITY ORGANIC CHEM FACOTRY
Hydrogen peroxide/cation resin system catalyzes the method of oxidizing alkenes to prepare vicinal diols
InactiveCN103992207BReduce pollutionMild reaction systemOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingOrganic synthesisCatalytic oxidation
The invention relates to a method for preparing vicinal diol by catalytic oxidation of alkene in a hydrogen peroxide / cationic resin system and belongs to the field of organic synthesis. The method is carried out by the following steps: adding alkene and cation exchange resin into a single-ported round-bottom flask; stirring and heating with a constant temperature oil bath pan at the temperature of 0-90 DEG C; measuring a hydrogen dioxide solution with a constant voltage dropping funnel according to the dosage of the added alkene; slowly adding a reaction solution after reaching a set temperature and reacting for 0.1-10h; adding a few amount of Na2S2O3 to quench the reaction after the end of the reaction; filtering out the cation exchange resin, cleaning with methanol and recovering; and extracting a reaction solution with ethyl acetate and water, putting a water phase into a refrigerator, and precipitating white crystals, namely a vicinal diol compound. The method requires no solvent; a catalyst can be recycled; a reaction system is mild; environmental pollutants are few; the method has a wide application range; and production cost is low. By optimizing reaction conditions, product purity is higher than 92%, and yield can reach 89%. The method has a strong industrial application prospect.
Owner:CHANGZHOU UNIV
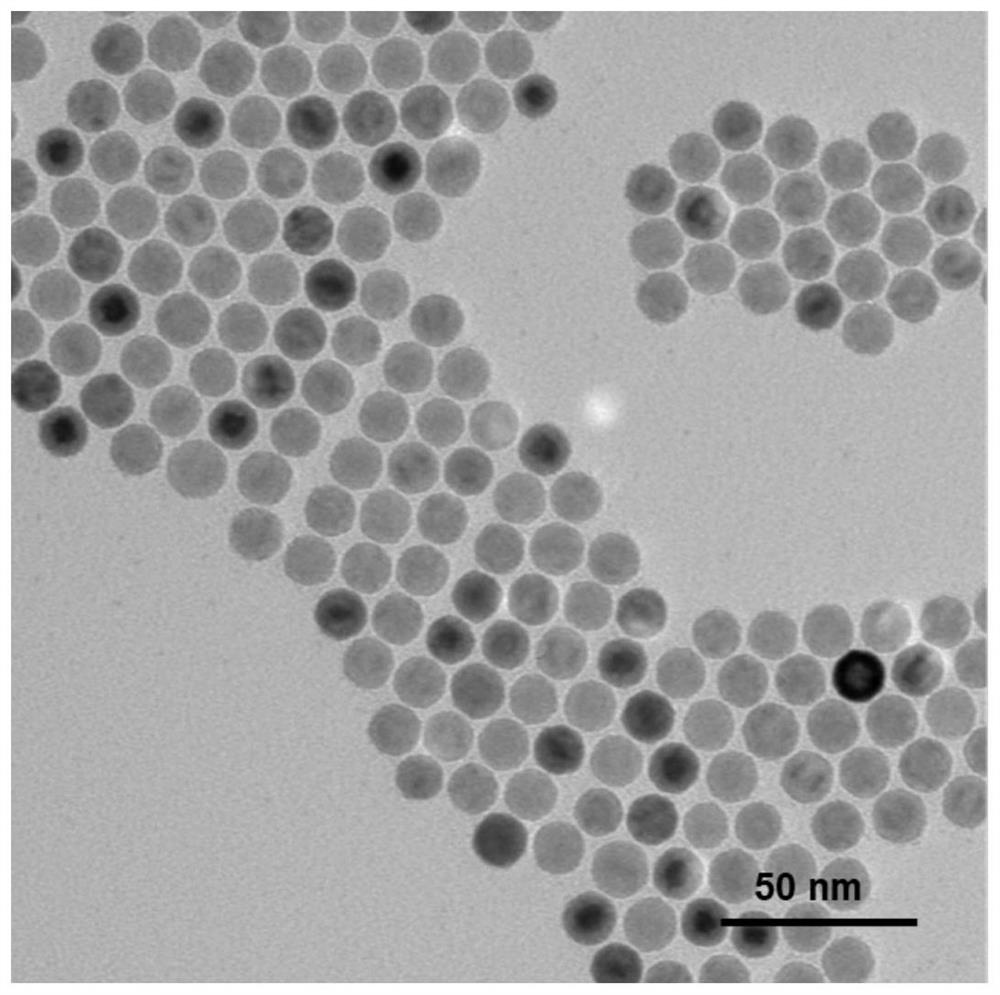
Preparation method, product and application of inorganic nanoparticle/supramolecular nano targeting compound
ActiveCN112007177AEnhance tumor targeting effectImprove colloidal stabilityEmulsion deliveryIn-vivo testing preparationsMedical imagingNanoparti cles
The invention discloses a preparation method of an inorganic nanoparticle / supramolecular nano targeting compound. The preparation method comprises the following steps: preparing uniform inorganic nanoparticles coated with hydrophobic ligands or cationic ligands; carrying out ligand exchange reaction with a polymer ligand with terminal carboxyl to obtain carboxyl-modified inorganic nanoparticles; carrying out amide condensation reaction on the inorganic nanoparticles and object small molecules with polyamine structures to obtain object molecule modified inorganic nanoparticles; carrying out host-guest interaction with macrocyclic supramolecules as a host to obtain supramolecule modified inorganic nanoparticles; and carrying out host-guest interaction again with a guested targeting ligand toobtain the inorganic nanoparticle / supramolecular nano targeting compound. The invention also discloses the compound obtained by the preparation method and application of the compound in preparation of a biomedical imaging nano contrast agent. According to the compound, targeting ligands are orderly arranged on the surfaces of nanoparticles, and the compound has excellent disease targeting and imaging effects.
Owner:ZHEJIANG UNIV
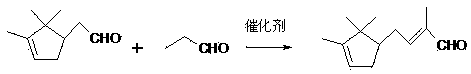
Production method of 2-campholenic propyl aldehyde
ActiveCN103449988ASolve the existence of difficult to purifySolve quality problemsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystReaction temperature
The invention discloses a production method of 2-campholenic propyl aldehyde. The production method of the 2-campholenic propyl aldehyde comprises the following steps: adding campholenic aldehyde, n-propanal, methanol or ethanol and a catalyst, namely proline or a derivative of the proline into a reaction kettle, performing direct reaction at a certain reaction temperature, and performing neutralization, washing with water and purification, thereby obtaining a 2-campholenic propyl aldehyde product. According to the synthesis production process provided by the invention, a one-pot method reaction technology is adopted, the proline catalyst is used for replacing a traditional strong base catalyst, byproducts brought by a traditional process are reduced, the product yield and the product quality are further improved, the environmental pollution is reduced, and the effects of reducing the production cost, and reducing the environmental protection cost are also achieved.
Owner:广州百花香料股份有限公司
A novel gossypol derivative and its preparation method and antitumor application
ActiveCN104817472BRetain active phenolic hydroxylImprove biological activityOrganic compound preparationCarboxylic acid amides preparationOrganic synthesisSolvent
The invention specifically discloses novel gossypol derivatives, and a preparation method and application thereof, belonging to the technical field of organic synthesis. According to the method, gossypol and isonitrile are used as raw materials and undergo a stirring reaction in a polar solvent at 70 to 80 DEG C for 24 and 36 h, then simple filtering and separating are carried out, and thus, a series of the gossypol derivative with modified aldehyde groups are prepared through one-step reaction. The preparation method and post-treatment are simple; a reaction system is mild; and the synthesized novel gossypol derivatives retain all the active phenolic hydroxyl groups unique to gossypol to a greatest extent, and it is estimated that all the excellent biological activities of gossypol are retatined and the problem of toxic aldehyde groups carried by gossypol is overcome. Most of the gossypol derivatives prepared in the invention have good anti-prostatic cancer activity and can be applied in the antineoplastic field.
Owner:江苏度未生物工程科技有限公司
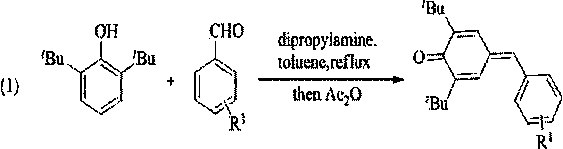
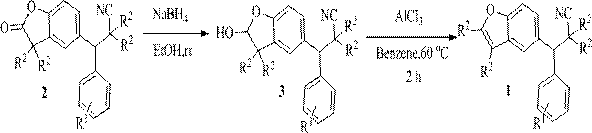
Synthetic method and application of cyanobenzofuran compound
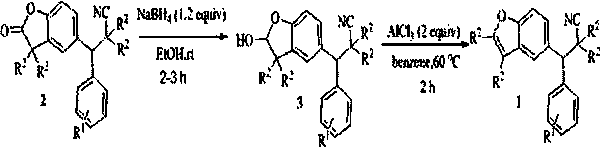
InactiveCN109851601ACheap and easy to getEasy to operateOrganic chemistryAntineoplastic agentsThin layer chromatographicOrganic layer
The invention discloses a synthetic method of a cyanobenzofuran compound. The synthetic method comprises the following steps: taking a benzofuran-2-ketone compound as a reaction initiator, taking ethyl alcohol as a solvent, slowly adding a reducing agent NaBH4 under a condition of 0 DEG C, then carrying out stirring for a reaction at room temperature, adding water for quenching, extracting the product by using ethyl acetate, then carrying out washing with saturated salt water, collecting the organic layer, carrying out drying and concentrating, carrying out column chromatography or thin-layerchromatography to obtain an intermediate benzofuran-2-ol, taking the benzofuran-2-ol as a raw material, AlCl3 as an additive, and benzene as a solvent to carrying out stirring for a reaction for 2 hours at 60 DEG C in a nitrogen environment, adding water for quenching, extracting the obtained product by using ethyl acetate, collecting the organic layer, carrying out drying and concentrating, and carrying out column chromatography or thin-layer chromatographic separation purification to obtain the target product. The synthesis reaction is simple to operate, the adopted reagents are cheap and are easily available, the reaction system is mild, the conditions are simple, the yield is good, and the method has relatively good popularization and application values.
Owner:JISHOU UNIVERSITY
Isoquinoline-1,3(2h,4h)-dione containing cycloalkylnitrile group and its production method and use
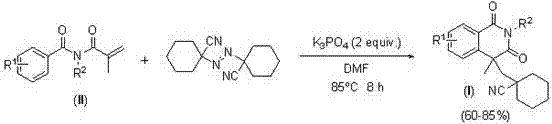
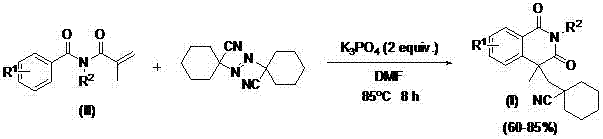
InactiveCN105859618BEasy to operateSimple conditionsOrganic chemistryUrinary disorderProstate cancer cellSource material
The invention discloses an isoquinoline-1,3(2H,4H)-diketone compound containing a naphthenic nitrile group, and a preparation method and use of the isoquinoline-1,3(2H,4H)-diketone compound. The isoquinoline-1,3(2H,4H)-diketone compound has a structure as shown in formula (I); the preparation method takes N-isobutylene acyl-N-alkyl benzamide as a reaction starting material, 1,1'-azobis dicyclohexyl nitrile as a free radical source material, K3PO4 as alkali and dimethyl formamide (DMF) as a solvent, and comprises the steps of carrying out a reaction at the temperature of 85 DEG C in an air environment; after the reaction is carried out for 8 hours, separating and purifying by column chromatography or thin-layer chromatography to obtain the isoquinoline-1,3(2H,4H)-diketone compound. The compounds has a very high inhibition effect for prostate cancer cells (LNCap cells), and particularly, one or two of the compounds are very promising, so that an excellent opportunity is provided for finding out a lead compound for treating prostatic cancer; furthermore, the preparation method is simple in operation of a synthesis reaction, does not need any metal catalyst, and is mild in reaction system, simple in conditions, low in cost and high in yield, thus having great popularization and application values.
Owner:JISHOU UNIVERSITY
A kind of cerium oxide/iron oxide/mesoporous silicon nanocomposite material and its preparation method and application
ActiveCN106860427BGood biocompatibilityGood potential for clinical translationNervous disorderInorganic non-active ingredientsNMR - Nuclear magnetic resonanceMagnetic resonance imaging contrast medium
The invention relates to a cerium oxide / iron oxide / mesoporous silica nano composite material. The surfaces of amino-modified mesoporous silica nanoparticles are respectively modified by ligand-converted cerium oxide nanocrystals and ligand-converted iron oxide nanocrystals. The invention also relates to a preparation method and application of the cerium oxide / iron oxide / mesoporous silica nano composite material. Not only can the composite material carry a drug for treating Alzheimer apostrophe s disease (AD), but also can remove reactive oxide species (ROS) in AD-similar cells, and can be used for diagnosis of the AD as a nuclear magnetic resonance imaging contrast agent, thus realizing integration of diagnosis and treatment of the AD.
Owner:ZHEJIANG UNIV
Method for synthesizing indolizine compound under catalysis of silver
ActiveCN113200980AEasy to separate and purifyEasy to operateOrganic chemistryChemical recyclingPtru catalystOrganic synthesis
The invention discloses a method for synthesizing indolizine compounds under the catalysis of silver, which comprises the following steps of in an organic solvent system, stirring N-benzoylmethyl pyridinium bromide as shown in a formula (1) and an isocyanide compound as shown in a formula (2) according to a feeding molar ratio of 1: (1.2-2.0) in the presence of a metal silver salt as a catalyst to react in the air under an alkaline condition, and carrying out TLC tracking detection until the reaction is complete, and carrying out post-treatment on the reaction liquid to obtain the indolizine compound as shown in a formula (3). The method is easy to operate, raw materials and reagents are easy to obtain, reaction conditions are mild, a reaction system is environmentally friendly, products are easy to separate and purify, the yield reaches up to 91%, and the method is suitable for efficient and high-yield preparation of indolizine compounds and particularly suitable for synthesis of various 1, 2-substituted indolizine compounds. The method is suitable for large-scale industrial production, and has wide application prospects and important significance in organic synthesis.
Owner:XUZHOU NORMAL UNIVERSITY
Preparation method, product and application of a pH-responsive nano-silver assembly
ActiveCN108721248BMild reaction systemConditions are easy to controlAntibacterial agentsInorganic active ingredientsSolventOleylamine
The invention relates to a preparation method of a pH-responsive nanosilver assembly. The preparation method comprises the following steps: 1), dissolving silver nitrate in oleylamine and oleic acid,heating for a reduction reaction, and precipitating by using a poor solvent to obtain silver nanoparticles; 2), dissolving an amphiphilic polymer in a good solvent, adding a modifier having a pH-responsive group, stirring at room temperature for a reaction, and dialyzing to obtain a pH-responsive amphiphilic polymer; 3), by a film dispersion method or an emulsion solvent evaporation method, assembling the silver nanoparticles obtained in the step 1) into the pH-responsive amphiphilic polymer obtained in the step 1) to obtain the pH-responsive nanosilver assembly. The invention further relatesto the pH-responsive nanosilver assembly prepared by the preparation method and application thereof. The pH-responsive nanosilver assembly has good antibacterial performance, not only can effectivelyinhibit growth of medicine-resistant bacteria, but also has the effects of inhibiting and destroying a bacterial biofilm.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com