Method for synthesizing indolizine compound under catalysis of silver
A compound, the technology of indolizine, which is applied in the field of silver-catalyzed synthesis of indolizine compounds, can solve the problems of inconvenient operation, high temperature and high pressure, and low yield, and achieve the effects of simple operation, mild reaction conditions, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of indolizine derivative 3a
[0034]
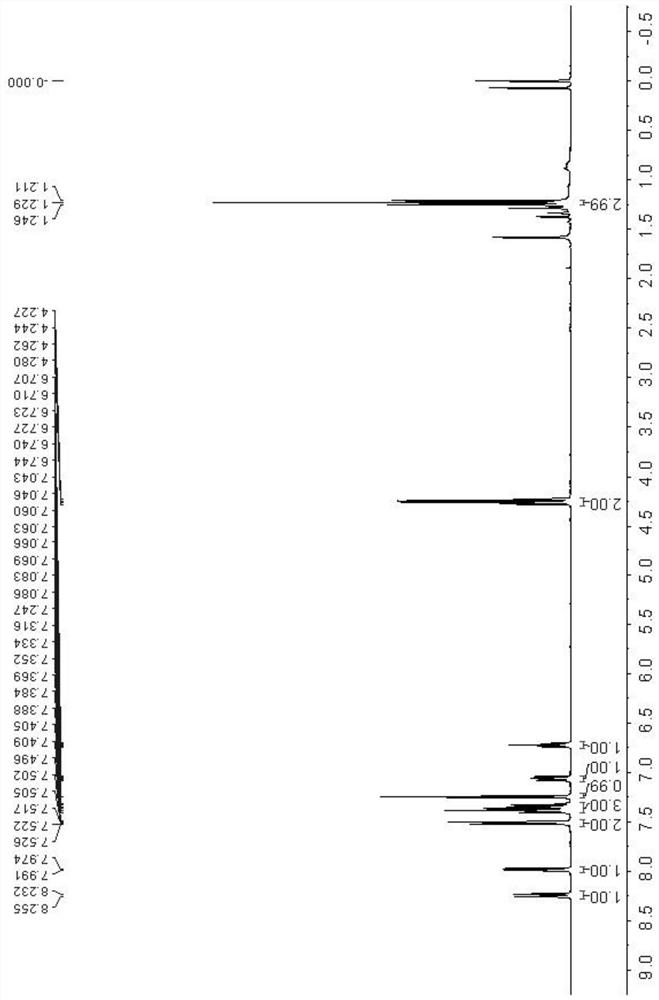
[0035] Add N-phenacylmethylpyridine bromide 1a (0.139 g, 0.5 mmol), silver carbonate (0.207 g, 0.75 mmol), triethylamine (104 μL, 0.75 mmol) into a 10 mL pressure-resistant tube with a magnetic stirring device , 1,4-dioxane (3 mL) and ethyl isocyanacetate 2a (66 μL, 0.6 mmol), stirred at 20° C. for 2 h. TLC (developing agent is V 石油醚 :V 乙酸乙酯 =10:1) The detection substrate disappears, and the reaction ends. The reaction solution was poured into a round-bottomed flask, and after distillation under reduced pressure, it was subjected to silica gel column chromatography (eluent was V 石油醚 :V 乙酸乙酯 =15:1) separation, the resulting eluate was distilled under reduced pressure and dried to obtain a white solid, which was confirmed to be indolizine derivative 3a by NMR and MS, with a yield of 88%.
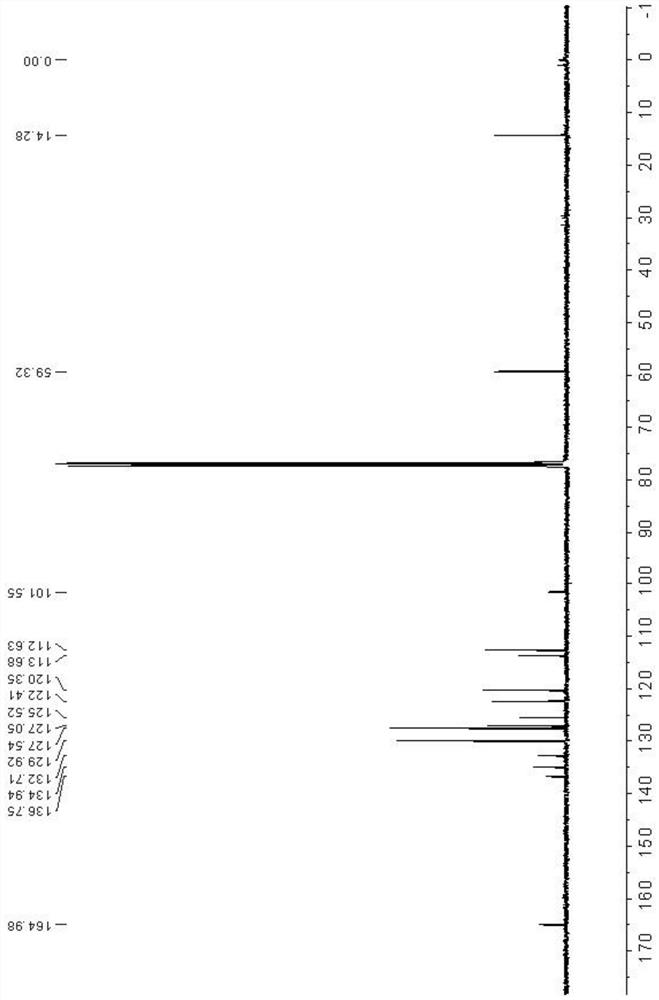
[0036] Through hydrogen spectrum, carbon spectrum and high-resolution mass spectrometry detection of indoliz...
Embodiment 2
[0039] Indolizine derivative 3b was prepared by replacing 1a in Example 1 with 1b.
[0040]
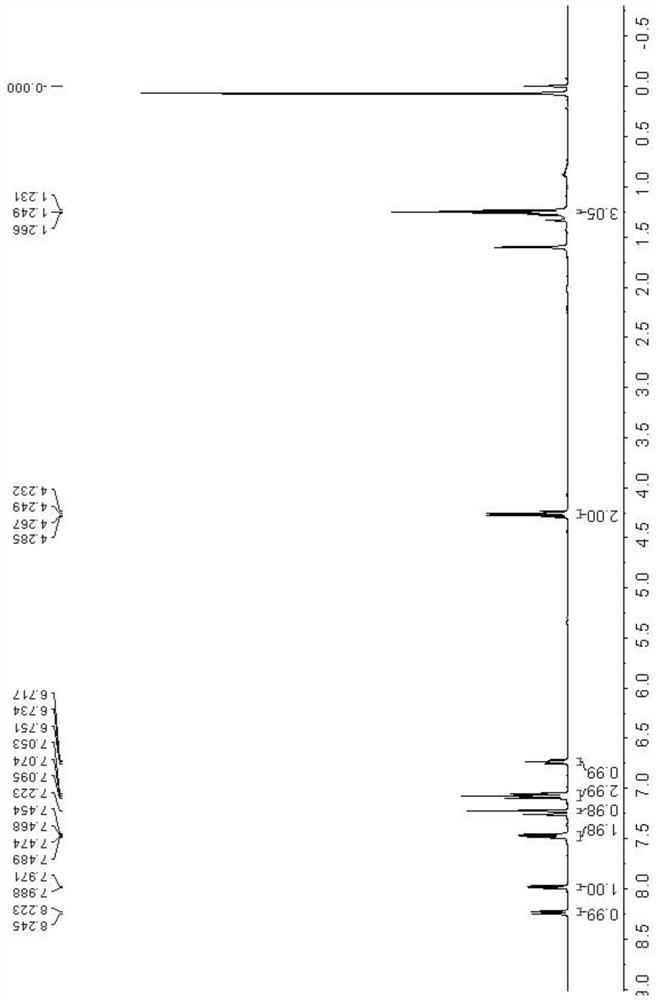
[0041] Add N-phenacylmethylpyridine bromide 1b (0.148g, 0.5mmol), silver carbonate (0.276g, 1.0mmol), potassium carbonate (0.104g, 0.75mmol) into a 10mL pressure-resistant tube with a magnetic stirring device , 1,4-dioxane (3 mL) and ethyl isocyanacetate 2a (66 μL, 0.6 mmol), stirred at 20° C. for 2 h. TLC (developing agent is V 石油醚 :V 乙酸乙酯 =10:1) The detection substrate disappears, and the reaction ends. The reaction solution was poured into a round-bottomed flask, and after distillation under reduced pressure, it was subjected to silica gel column chromatography (eluent was V 石油醚 :V 乙酸乙酯 =15:1) separation, the resulting eluate was distilled under reduced pressure and dried to obtain a white solid, which was confirmed to be indolizine derivative 3b by NMR and MS, with a yield of 86%.
[0042]Through the hydrogen spectrum, carbon spectrum and high-resolution mass spectrum dete...
Embodiment 3
[0045] Indolizine derivative 3c was prepared by substituting 1c for 1a in Example 1.
[0046]
[0047] Add N-phenacylmethylpyridine bromide 1c (0.156 g, 0.5 mmol), silver carbonate (0.207 g, 0.75 mmol), triethylamine (104 μL, 0.75 mmol) into a 10 mL pressure-resistant tube with a magnetic stirring device , 1,2-dichloroethane (2 mL) and ethyl isocyanacetate 2a (66 μL, 0.6 mmol), stirred at 20° C. for 2 h. TLC (developing agent is V 石油醚 :V 乙酸乙酯 =10:1) The detection substrate disappears, and the reaction ends. The reaction solution was poured into a round-bottomed flask, and after distillation under reduced pressure, it was subjected to silica gel column chromatography (eluent was V 石油醚 :V 乙酸乙酯 =15:1) separation, the resulting eluate was distilled under reduced pressure and dried to obtain a white solid, which was confirmed to be indolizine derivative 3c by NMR and MS, with a yield of 91%.
[0048] Through the hydrogen spectrum, carbon spectrum and high-resolution mass sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com