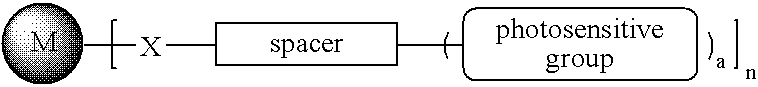
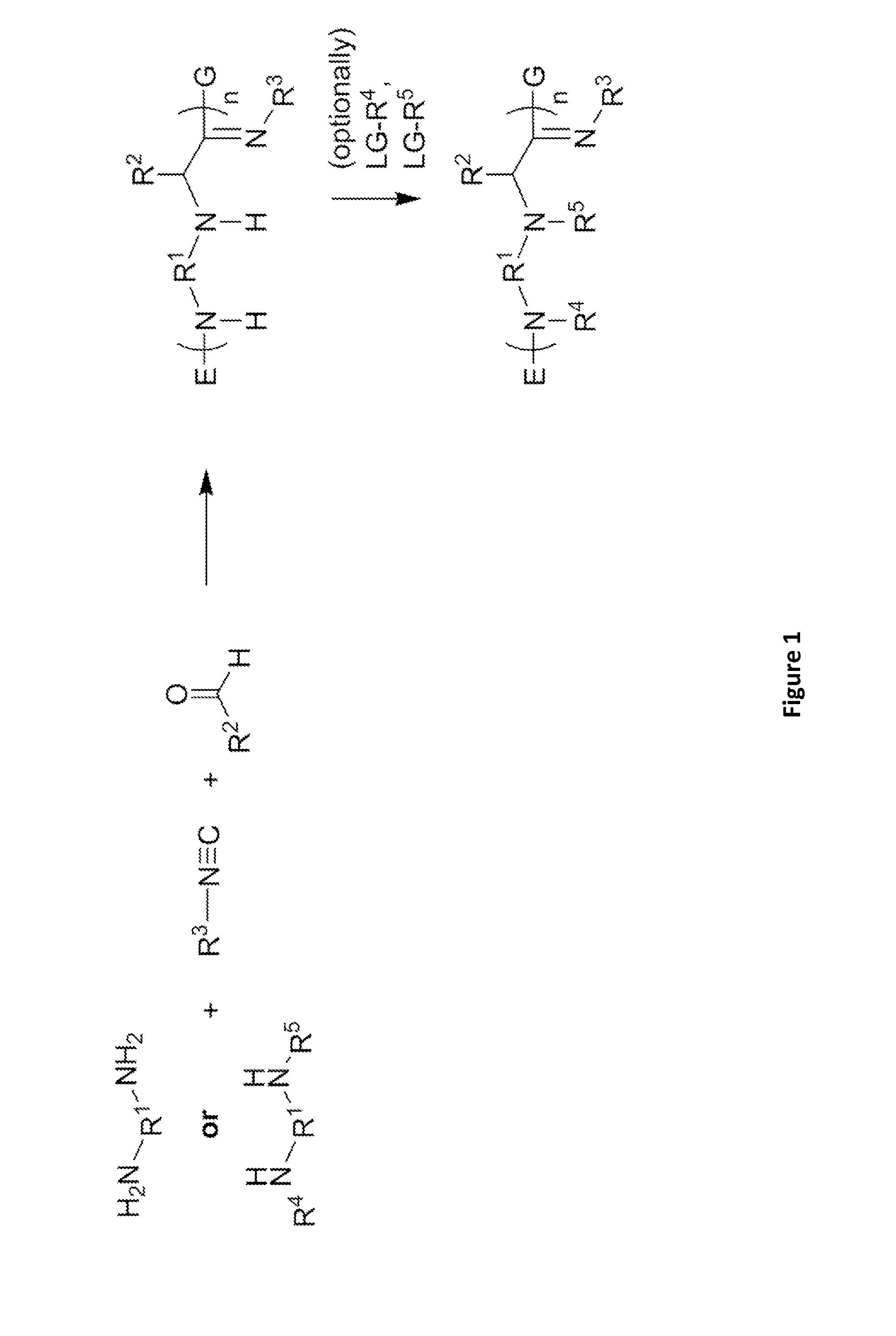
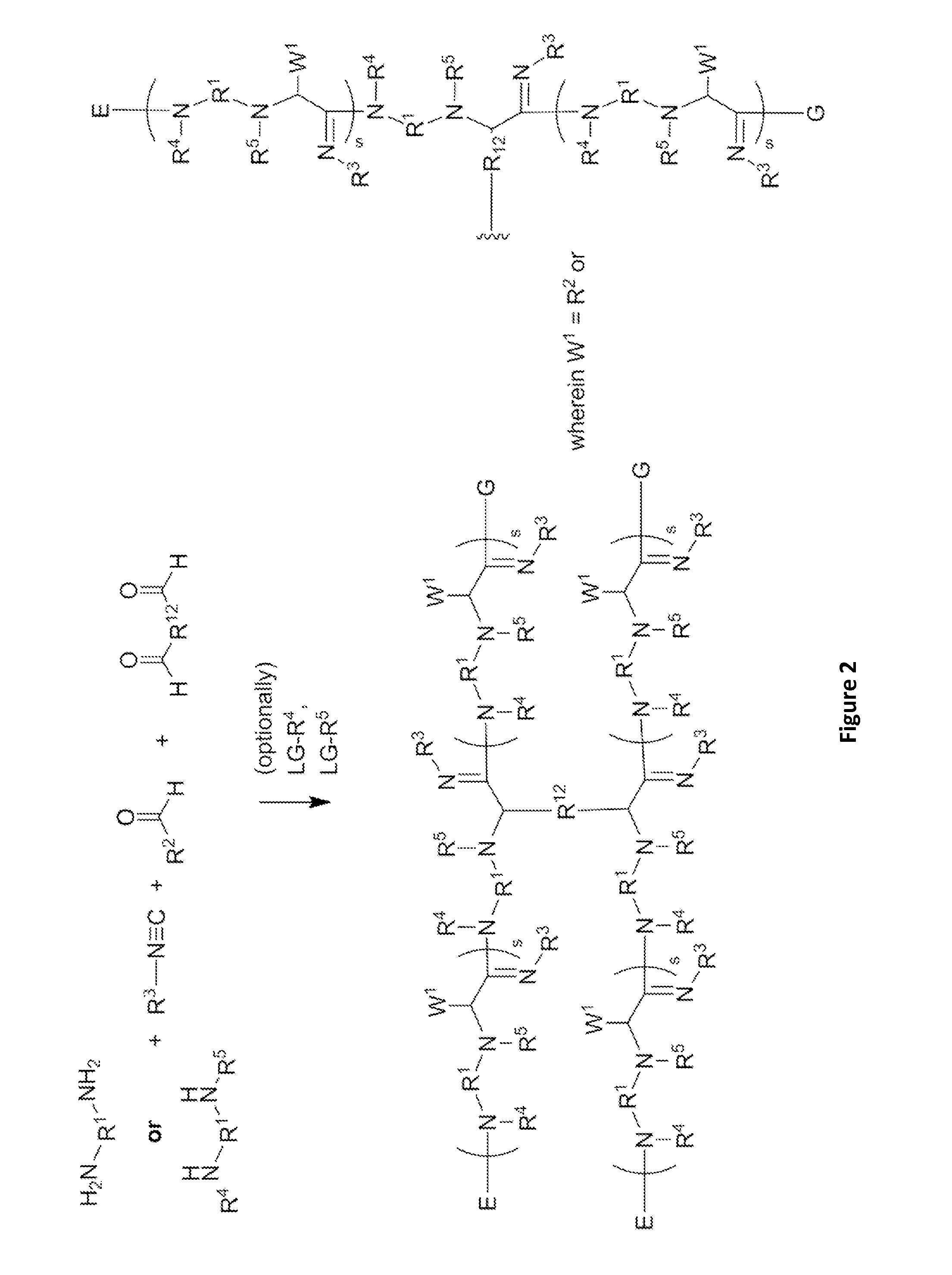
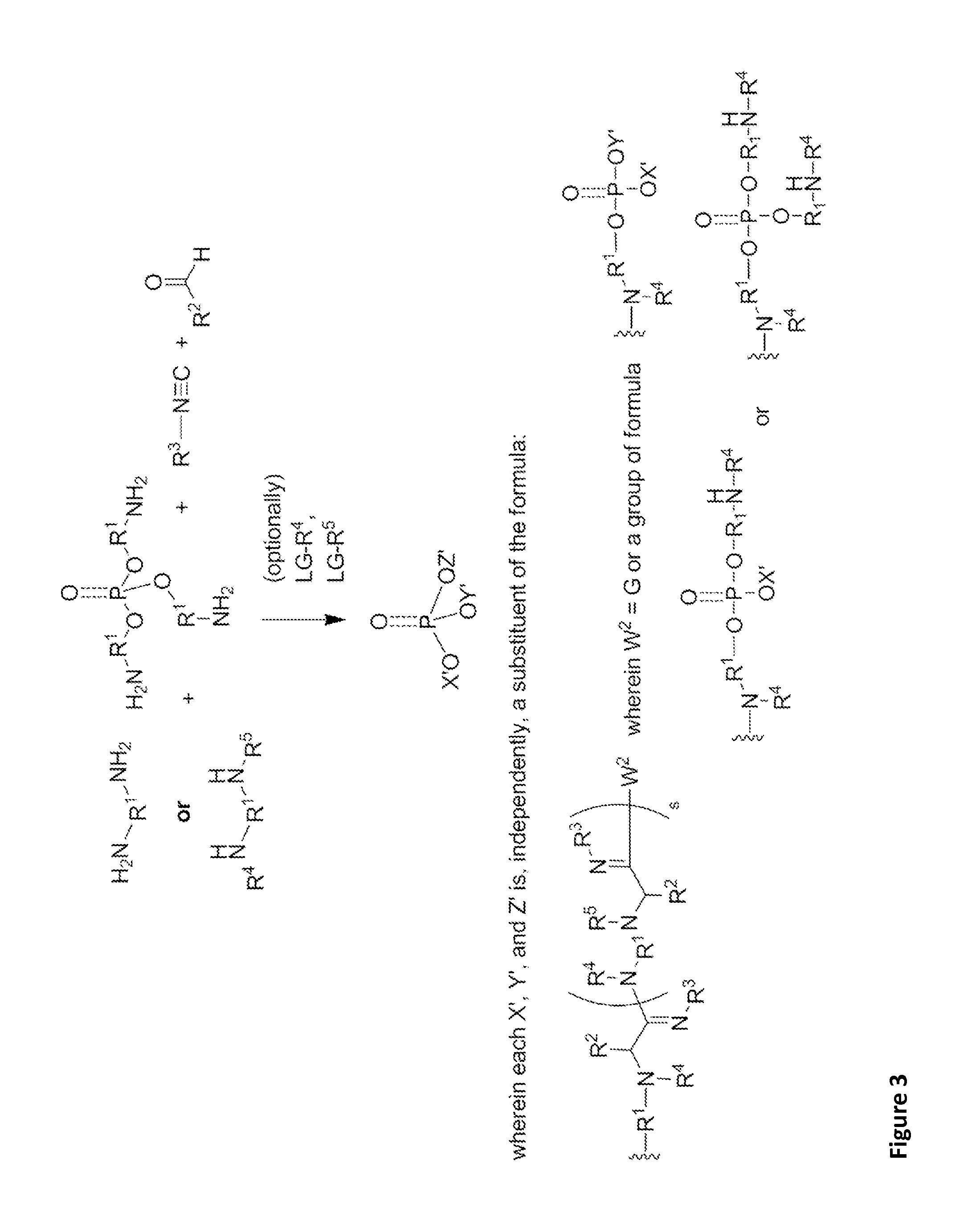
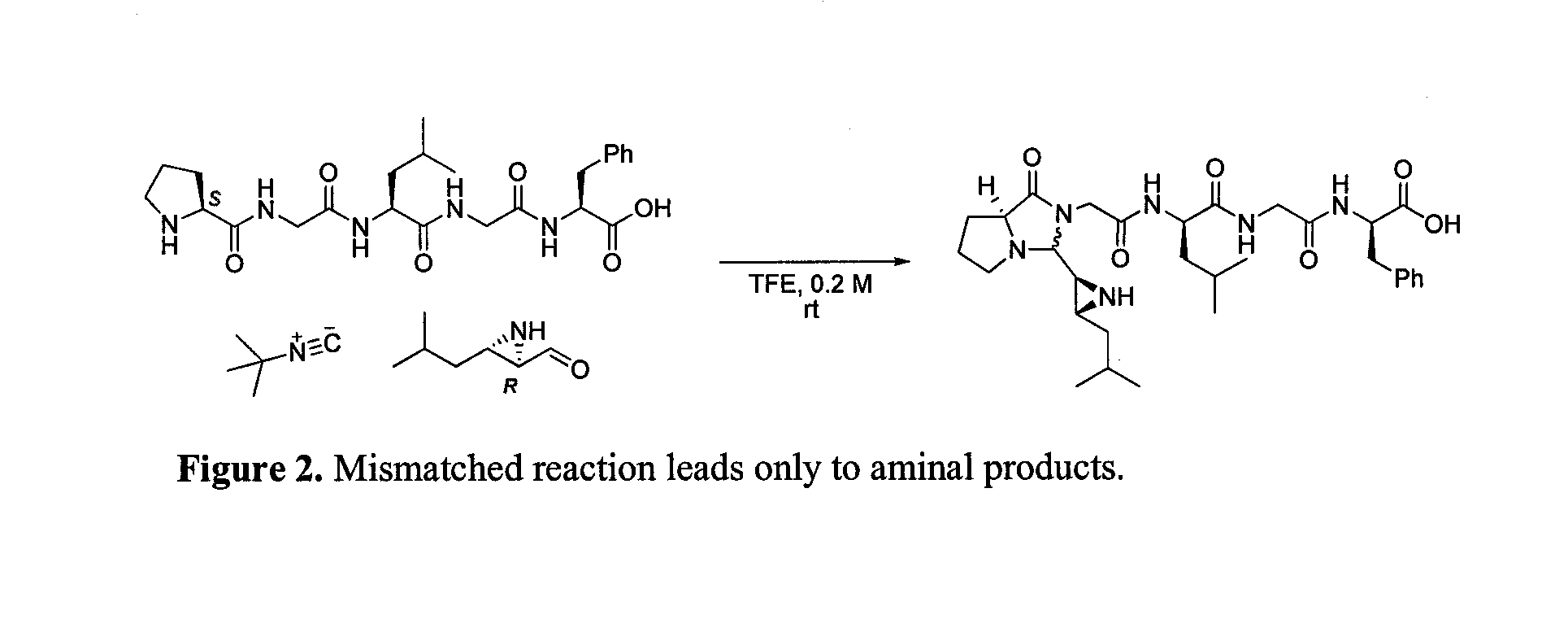
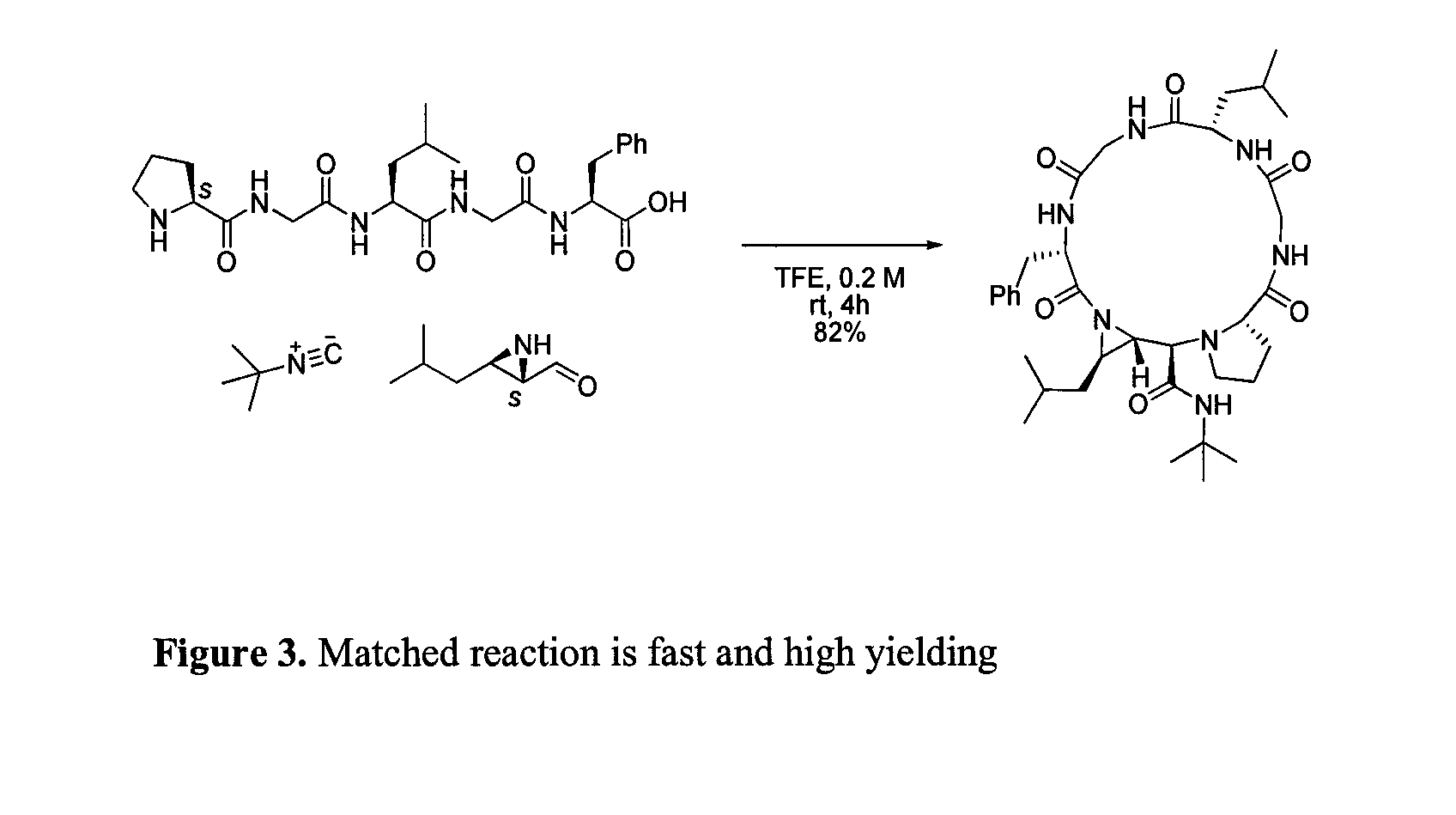
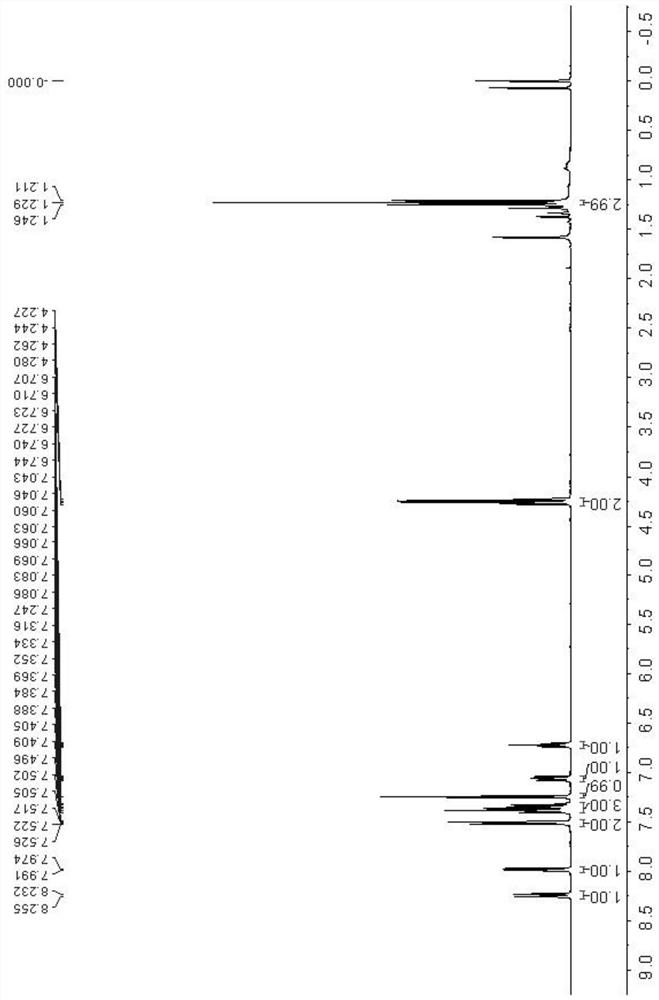
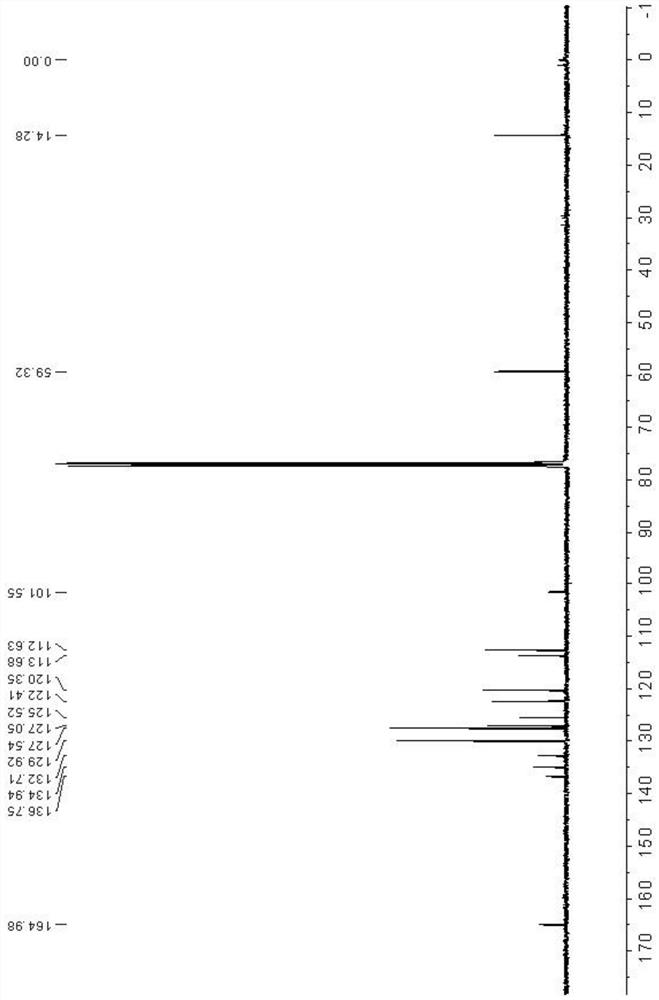
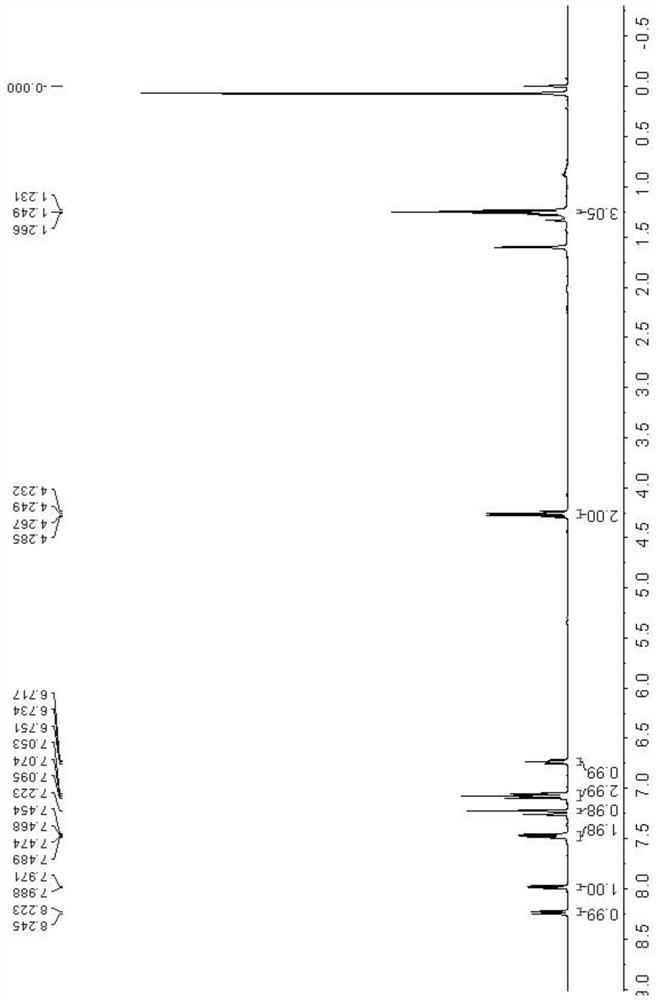
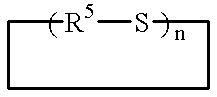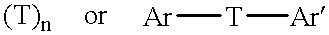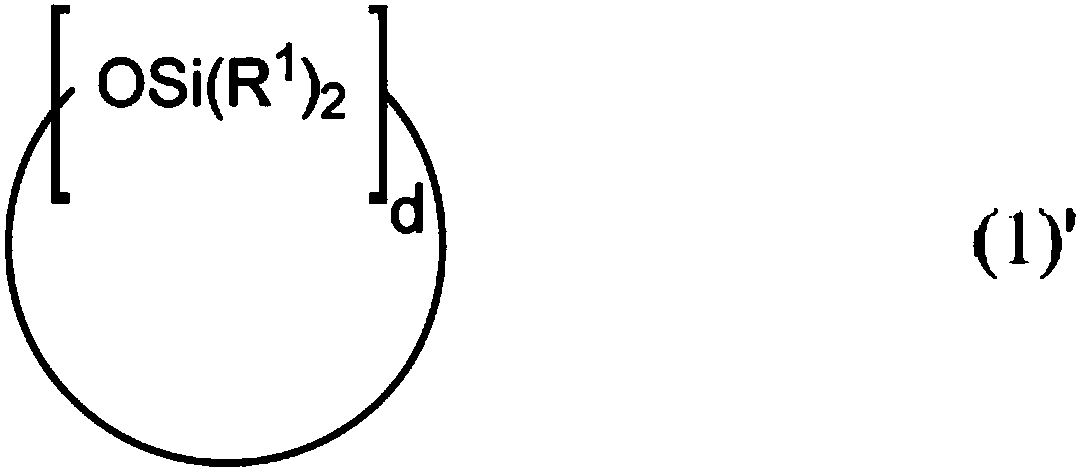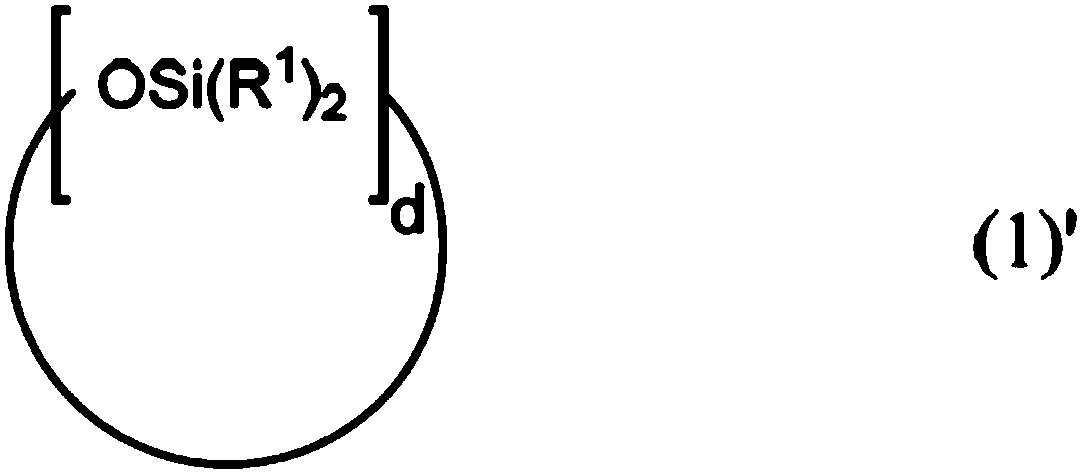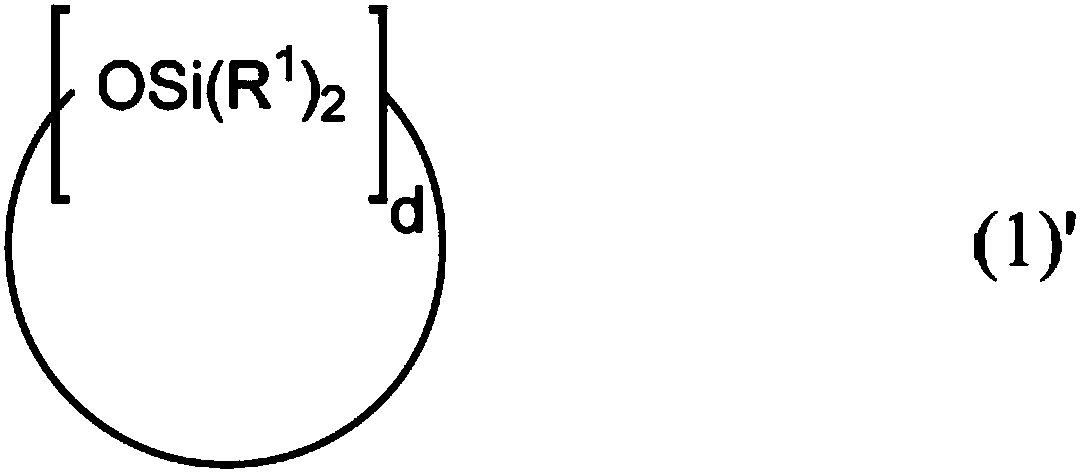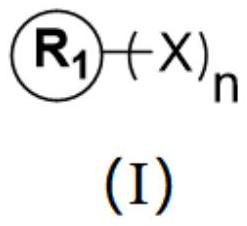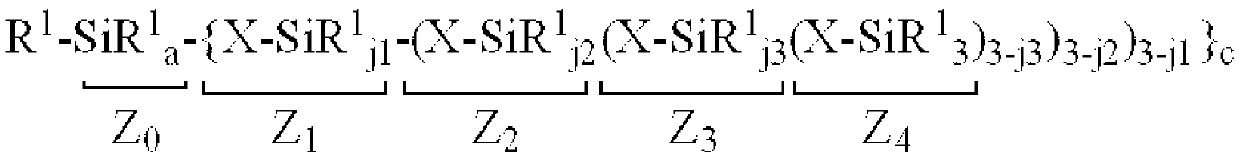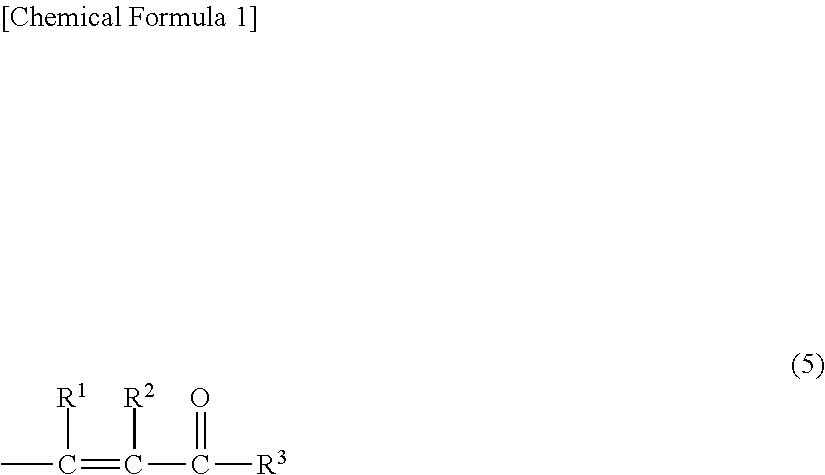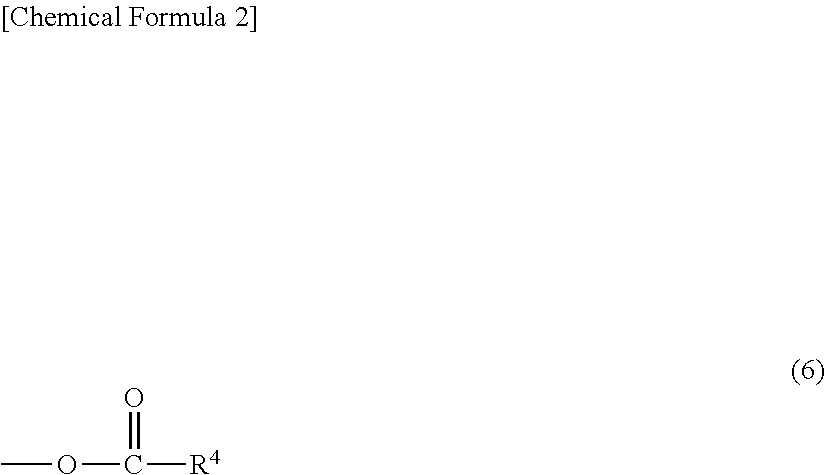Patents
Literature
49 results about "Isocyanide compound" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Photosensitive metal nanoparticle and method of forming conductive pattern using the same
ActiveUS7166412B2Easy to preparePattern of the metal nanoparticles can be easilyMaterial nanotechnologyNanostructure manufactureThiolSelf-assembled monolayer
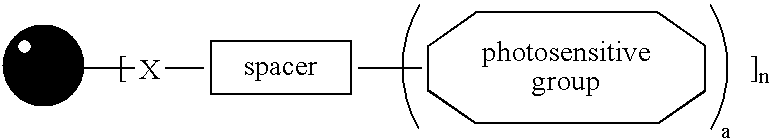
A photosensitive metal nanoparticle and a method of forming a conductive pattern using the same, wherein a self-assembled monolayer of a thiol compound or isocyanide compound having a terminal reactive group is formed on a surface of the metal nanoparticle and a photosensitive group is introduced to the terminal reactive group. The photosensitive metal nanoparticles can easily form a conductive film or pattern having excellent conductivity upon exposure to UV, and thus can be applied for antistatic washable sticky mats or shoes, conductive polyurethane printer rollers, electromagnetic interference shielding, etc.
Owner:SAMSUNG ELECTRONICS CO LTD
Method for preparing polythiourea through multi-component polymerization of isocyanide, sulfur and amine and application of polythiourea
InactiveCN107236128AHigh molecular weightAtom economy is highFluorescence/phosphorescenceThioureaFluorescence
The invention belongs to the technical field of preparation of sulfur-containing organic polymers and discloses a method for preparing a polythiourea through multi-component polymerization of isocyanide, sulfur and amine and application of the polythiourea. The preparation method comprises, reacting amine monomers, isocyanide monomers and elemental sulfur in solvent, and performing cooling, precipitation and drying processes to obtain the polythiourea, wherein the amine monomers are diamine compounds, and the isocyanide monomers are di-isocyanide compounds. The method for preparing the polythiourea through multi-component polymerization of isocyanide, sulfur and amine can be implemented at room temperature and in air, thereby being simple, high in polymerization reaction yield and atom economy and simple in product separation; the prepared polythiourea is rich in heteroatoms of N and S and obtains special photoelectric properties, thereby obtaining potential unique application value in fields of biological and chemical fluorescence detection and mercury ion detection.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing polythiourea by means of polymerizing multiple components of isocyanide, sulfur and amine and application of polythiourea
ActiveCN108570149AHigh molecular weightAtom economy is highFluorescence/phosphorescenceThioureaFluorescence
The invention belongs to the technical field of sulfur-containing organic polymer preparation, and discloses a method for preparing polythiourea by means of polymerizing multiple components of isocyanide, sulfur and amine and application of the polythiourea. The method includes carrying out reaction on amine monomers, isocyanide monomers and elemental sulfur in solvents; cooling, precipitating anddrying reaction products to obtain the polythiourea. The amine monomers are diamine compounds, and the isocyanide monomers are binary isocyanide compounds. The method and the application have the advantages that the method can be implemented under room-temperature and air conditions, is simple and is high in polymerization reaction yield and atom economy, and the polythiourea which is a product is easy to separate; the polythiourea prepared by the aid of the method contains abundant N and S heteroatoms, has special photoelectric properties and has a potential unique application value in the field of biological and chemical fluorescence detection, metallic mercury ion detection and mercury ion removal; the polythiourea is high in metallic mercury ion detection sensitivity, good mercury ionremoval effects can be realized by the polythiourea, and the removal efficiency of the polythiourea is higher than 99.99% and can reach drinking water standards.
Owner:SOUTH CHINA UNIV OF TECH
Photoresist composition for negative development and pattern forming method using thereof
InactiveUS20120122031A1Negative developmentPhotosensitive materialsPhotomechanical exposure apparatusResistLithographic artist
The present invention relates to a photoresist composition capable of negative development and a pattern forming method using the photoresist composition. The photoresist composition includes an imaging polymer and a radiation sensitive acid generator. The imaging polymer includes a first monomeric unit having a pendant acid labile moiety and a second monomeric unit containing a reactive ether moiety, an isocyanide moiety or an isocyanate moiety. The patterning forming method utilizes an organic solvent developer to selectively remove unexposed regions of a photoresist layer of the photoresist composition to form a patterned structure in the photoresist layer. The photoresist composition and the pattern forming method are especially useful for forming material patterns on a semiconductor substrate using 193 nm (ArF) lithography.
Owner:GLOBALFOUNDRIES INC
Photosensitive metal nanoparticle and method of forming conductive pattern using the same
InactiveUS7473513B1Easy to prepareMaterial nanotechnologyNanostructure manufactureCyanide compoundNanoparti cles
Owner:SAMSUNG ELECTRONICS CO LTD
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20050137400A1Discharge tube luminescnet screensElectroluminescent light sourcesCyanide compoundPhenanthroline
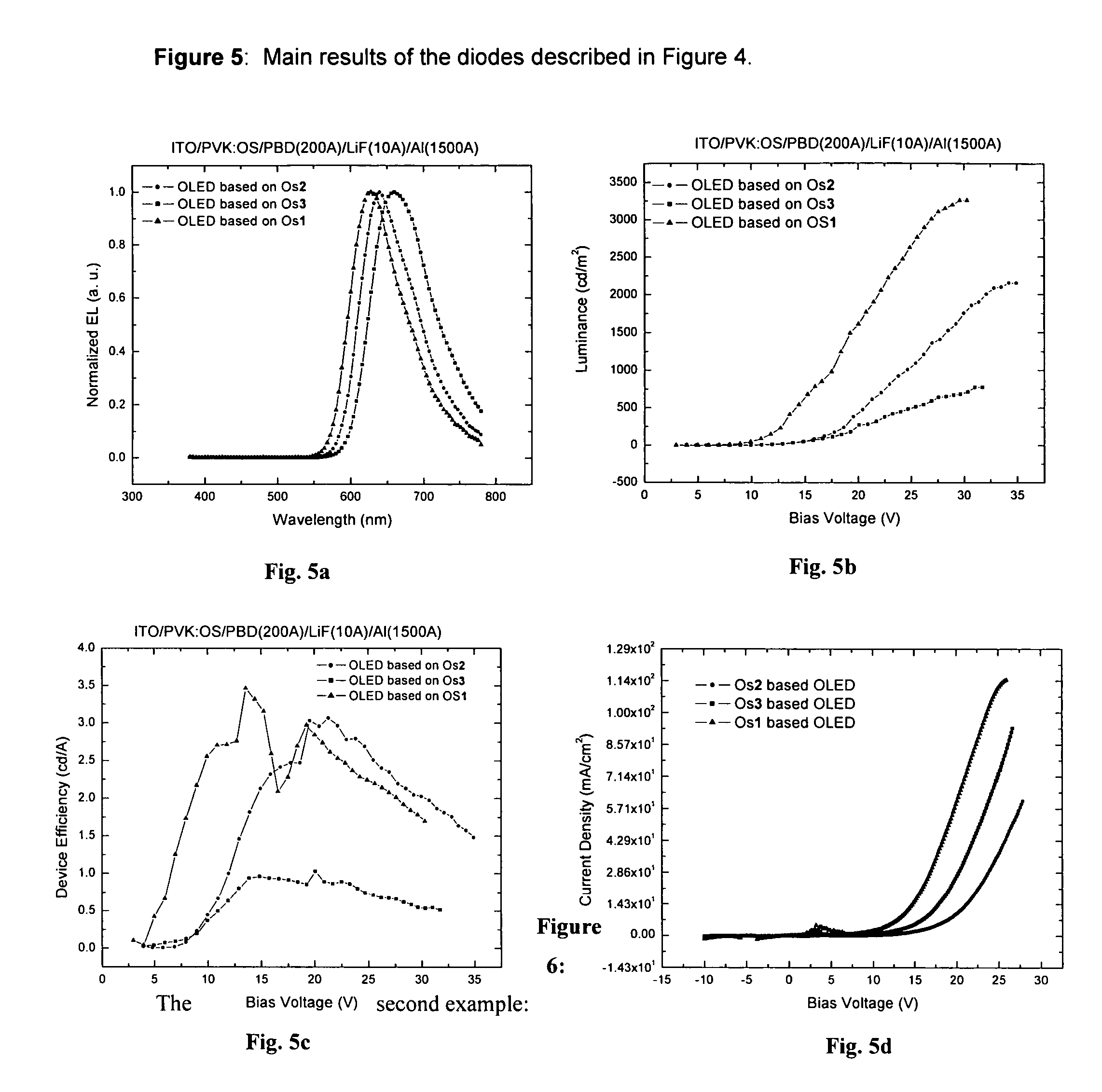
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores N{circumflex over ( )}N, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NAN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NAN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of N{circumflex over ( )}N chromophores residing opposite to the L. X1, X2 and X3 independently are C or N; when X2 is N, R, is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:NATIONAL TSING HUA UNIVERSITY +1
Addition-curing organopolysiloxane composition
An addition-curing organopolysiloxane composition which has excellent shelf stability around room temperature, rapidly cures by moderate ultraviolet irradiation or heating, and is suitable for use as the one solution type. A reaction inhibitor is added to the composition comprising an alkenylated organopolysiloxane, an organohydrogenpolysiloxane, and a platinum catalyst. The inhibitor is a thiophene compound, an isocyanide compound, a cyclic thioether compound, a cyclic azo ether compound, an imino compound, or a polymer which has, in the molecule, functional groups each containing one or more sulfur, nitrogen, or phosphorus atoms and has an average molecular weight of 1,000 to 200,000, and which is capable of forming a complex with a platinum atom.
Owner:THREE BOND CO LTD
Novel isocyanide compound and hydrosilylation reaction catalyst
ActiveCN108473514AImprove solubilityEasy to getSilicon organic compoundsOrganic chemistry methodsPlatinumPolymer science
Provided are a novel isocyanide compound, a hydrosilylation reaction catalyst having excellent handling properties and storage properties that allows a hydrosilylation reaction to proceed under moderate conditions by using the isocyanide compound, and a method for producing an addition compound by a hydrosilylation reaction using the hydrosilylation reaction catalyst. A hydrosilylation reaction catalyst prepared from a catalyst precursor comprising a transition metal compound of groups 8, 9, or 10 of the periodic table, excluding platinum, such as an iron carboxylate, cobalt carboxylate, or nickel carboxylate, and a ligand comprising an isocyanide compound having an organosiloxane group.
Owner:SHIN ETSU CHEM CO LTD +1
Photosensitive metal nanoparticle and method of forming conductive pattern using the same
ActiveUS20080311513A1Pattern of the metal nanoparticles can be easilyEasy to prepareMaterial nanotechnologyNanostructure manufactureCyanide compoundNanoparti cles
A photosensitive metal nanoparticle and a method of forming a conductive pattern using the same, wherein a self-assembled monolayer of a thiol compound or isocyanide compound having a terminal reactive group is formed on a surface of the metal nanoparticle and a photosensitive group is introduced to the terminal reactive group. The photosensitive metal nanoparticles can easily form a conductive film or pattern having excellent conductivity upon exposure to UV, and thus can be applied for antistatic washable sticky mats or shoes, conductive polyurethane printer rollers, electromagnetic interference shielding, etc.
Owner:SAMSUNG ELECTRONICS CO LTD
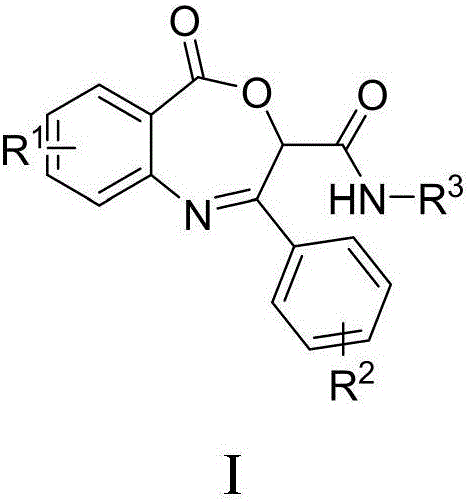
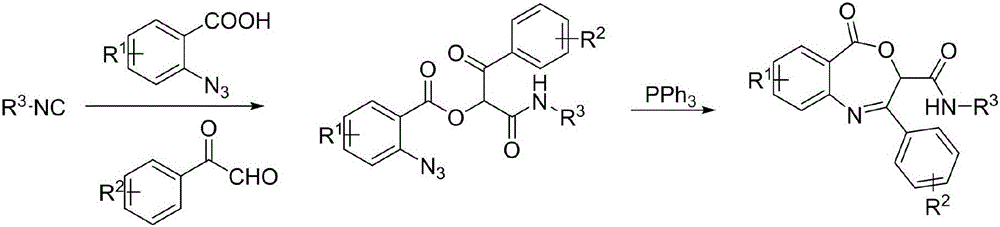
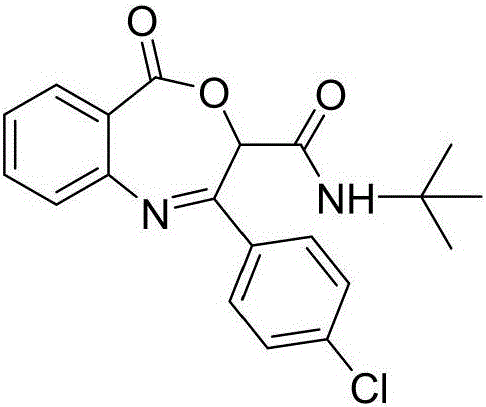
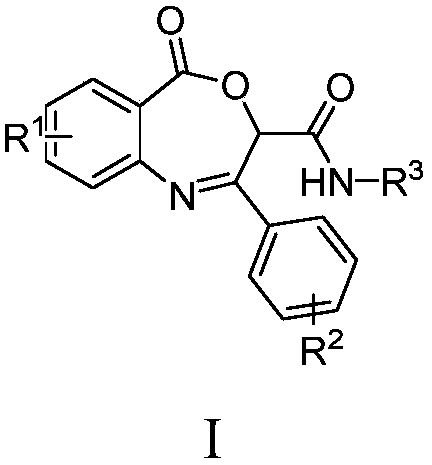
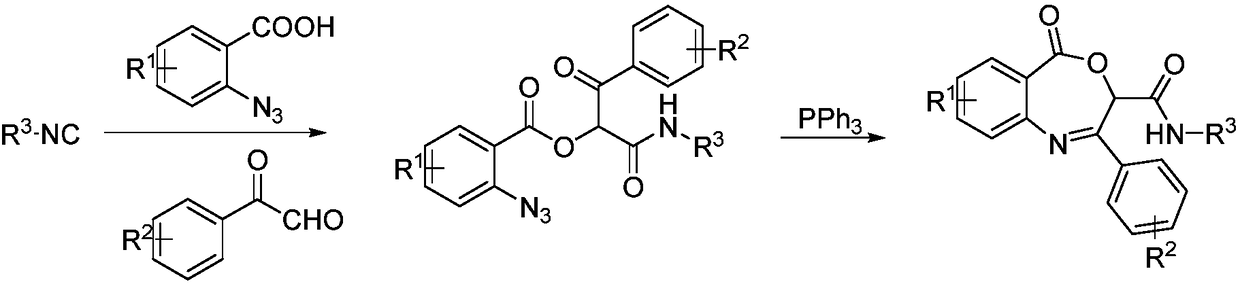
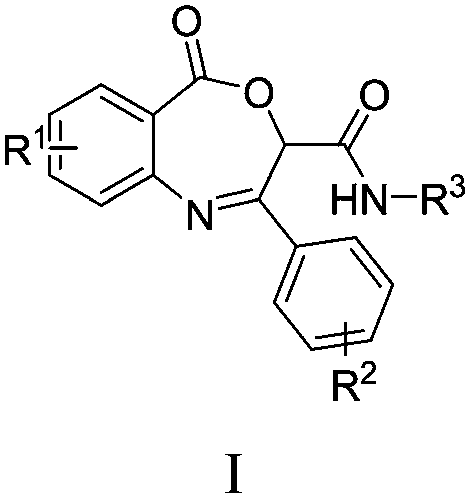
Benzoxazepin bacteriostats and synthetic method thereof
ActiveCN106831635AGood inhibitory effectReduce manufacturing costBiocideOrganic chemistryStructural formulaTert butyl
The invention relates to benzoxazepin bacteriostats and a preparation method thereof. The chemical structural formula is shown in the specification, wherein substituents R1, R2 and R3 are chloro, bromo, methyl, normal-butyl, p-chlorophenyl, methyl acetate groups, tertiary butyl or cyclohexyl, and positions, the number and conjugate positions of the substituents are not fixed. According to the synthetic method, ortho-azidobenzoic acid compounds and aryl ketoaldehyde compounds and in-situ formed isocyanide compounds are subjected toa Passerini reaction, a formed product and triphenylphosphine are subjected toa Staudinger reaction, and polysubstituted benzoxazepin heterocyclic compounds and other important heterocyclic compounds are produced through ring closing of an intramolecular aza-Wittig reaction. The key point of the invention is to provide one novel synthetic method which adopts fewer steps and has high yield to synthesize the novel benzoxazepin compounds. The compounds have better inhibitory activity on Rhizoctonia solani, Penicillium digitatum and Penicillium italicum and can be used as the bacteriostats.
Owner:CHINA THREE GORGES UNIV
Metal complex and supported metal complex having disiloxane as ligand, method for production therefof, and supported metal catalyst prepared by using the same
ActiveUS20160030932A1Maintain catalytic activityHigh catalytic activityAluminium compoundsHydrocarbon by hydrogenationDisiloxaneCarbene
A metal complex represented by the following Formula (1):(wherein M represents palladium or platinum; L represents a ligand selected from carbon monoxide, an olefin compound, an amine compound, a phosphine compound, an N-heterocyclic carbene compound, a nitrile compound and an isocyanide compound; n represents an integer of 0 to 2 showing the number of the ligand; and each of R1 to R4 represents an organic group). The metal complex described above can be fixed on an inorganic oxide while maintaining a skeletal structure thereof to obtain a supported metal complex, which makes it possible to allow the supported metal complex to maintain the same catalytic activity as that of the original metal complex. Also, calcining the supported metal complex obtained in the manner described above makes it possible to obtain a supported metal catalyst improved in catalytic activity to a greater extent than conventional supported metal catalysts.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Isocyanide compound and hydrosilylation reaction catalyst
ActiveUS10829504B2Eliminate needAllowing hydrosilylation reaction to take place effectivelySilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCyanide compoundPlatinum
Provided are a novel isocyanide compound, a hydrosilylation reaction catalyst having excellent handling properties and storage properties that allows a hydrosilylation reaction to proceed under moderate conditions by using the isocyanide compound, and a method for producing an addition compound by a hydrosilylation reaction using the hydrosilylation reaction catalyst.A hydrosilylation reaction catalyst prepared from a catalyst precursor comprising a transition metal compound of groups 8, 9, or 10 of the periodic table, excluding platinum, such as an iron carboxylate, cobalt carboxylate, or nickel carboxylate, and a ligand comprising an isocyanide compound having an organosiloxane group.
Owner:SHIN ETSU CHEM IND CO LTD +1
Hydrosilylation reaction catalyst
ActiveUS20170233417A1Easy to handleImprove responseSilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCobalt acetatePlatinum
A hydrosilylation reaction catalyst prepared from: a catalyst precursor comprising a transition metal compound, excluding platinum, belonging to group 8-10 of the periodic table, e.g., iron acetate, cobalt acetate, nickel acetate, etc.; and a ligand comprising an isocyanide compound such as t-butyl isocyanide. The hydrosilylation reaction catalyst has excellent handling and storage properties. As a result of using this catalyst, a hydrosilylation reaction can be promoted under gentle conditions.
Owner:KYUSHU UNIV +1
N-heterocyclic condensed tryptamine-beta-lactam derivative as well as preparation method and application thereof
InactiveCN111533732AHas antitumor activityEasy to operateOrganic chemistryAntineoplastic agentsCyanide compoundOrganic synthesis
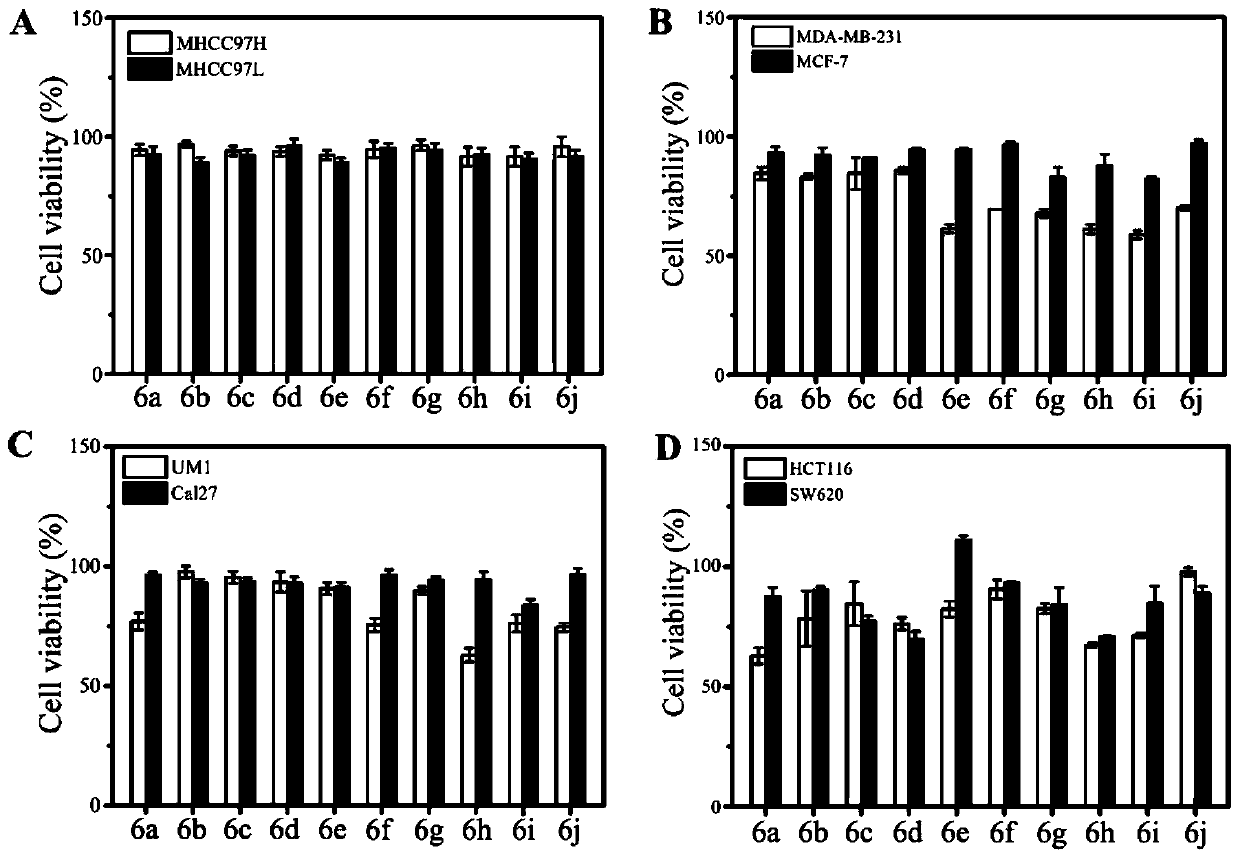
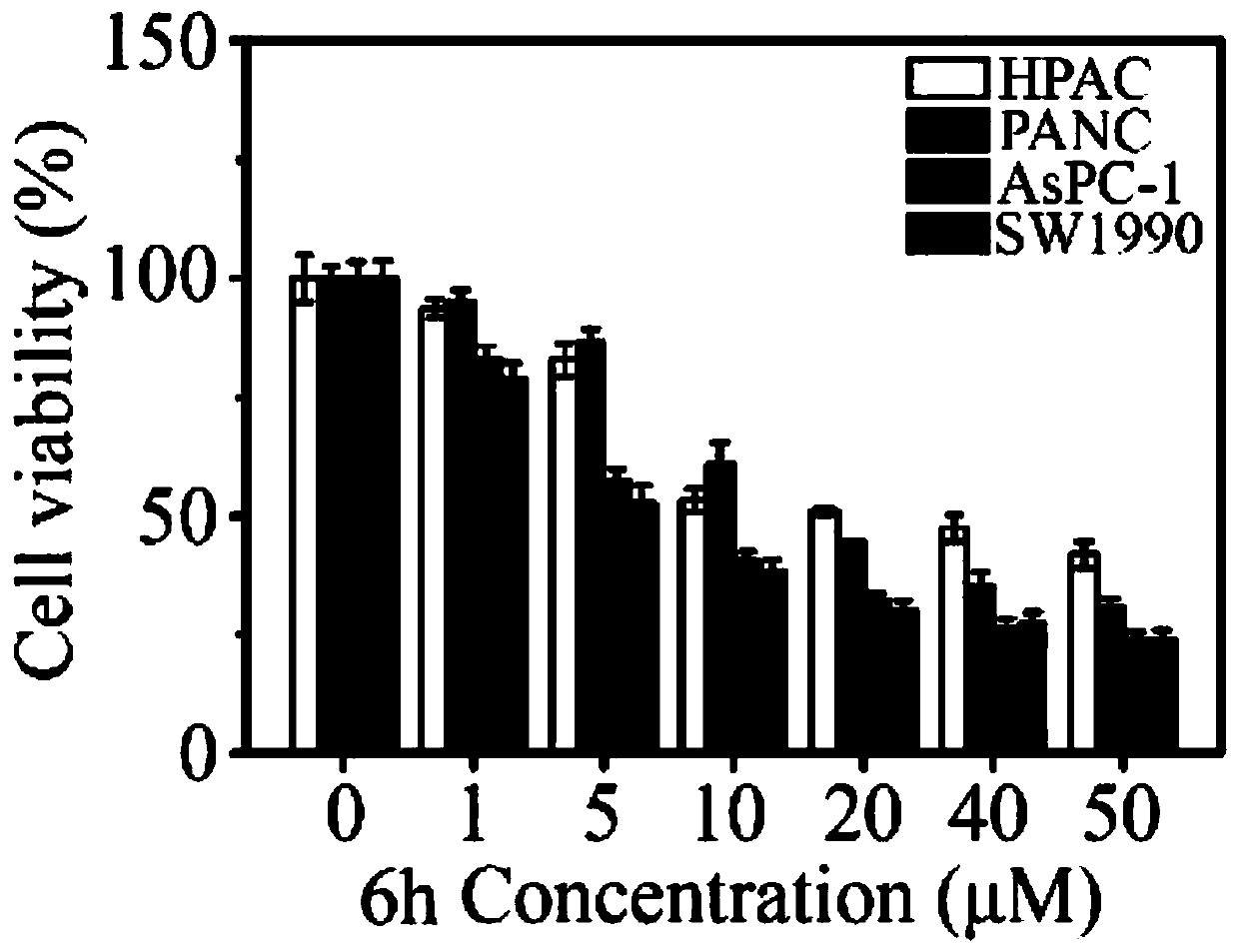
The invention relates to the technical field of organic synthesis, in particular to an N-heterocyclic condensed tryptamine-beta-lactam derivative as well as a preparation method and application thereof. The preparation method comprises the following steps: adding an aldehyde compound and propiolic acid into methanol and stirring; then adding tryptamine and isocyanide, and reacting under stirring;removing the methanol in a nitrogen atmosphere; dissolving obtained residues in acetonitrile, adding K2CO3, sealing the mixture, heating the mixture in a microwave oven, removing the acetonitrile, diluting with ethyl acetate, washing with saline water, drying with sodium sulfate, concentrating, purifying the obtained concentrate by silica gel column chromatography, and drying to obtain the N-heterocyclic condensed tryptamine-beta-lactam derivative. The N-heterocyclic fused tryptamine-beta-lactam derivative has a certain inhibition effect on the cell viability of liver cancer, breast cancer, head and neck squamous cell carcinoma and colon cancer cell lines, can inhibit or kill tumor cells, has antitumor activity, and can be applied to preparation of antitumor drugs.
Owner:CHONGQING UNIV OF ARTS & SCI
Preparation method for progesterone
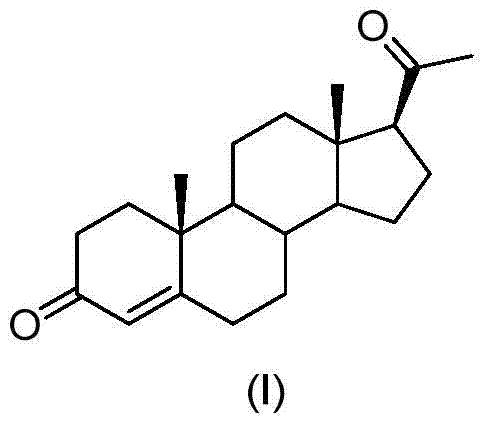
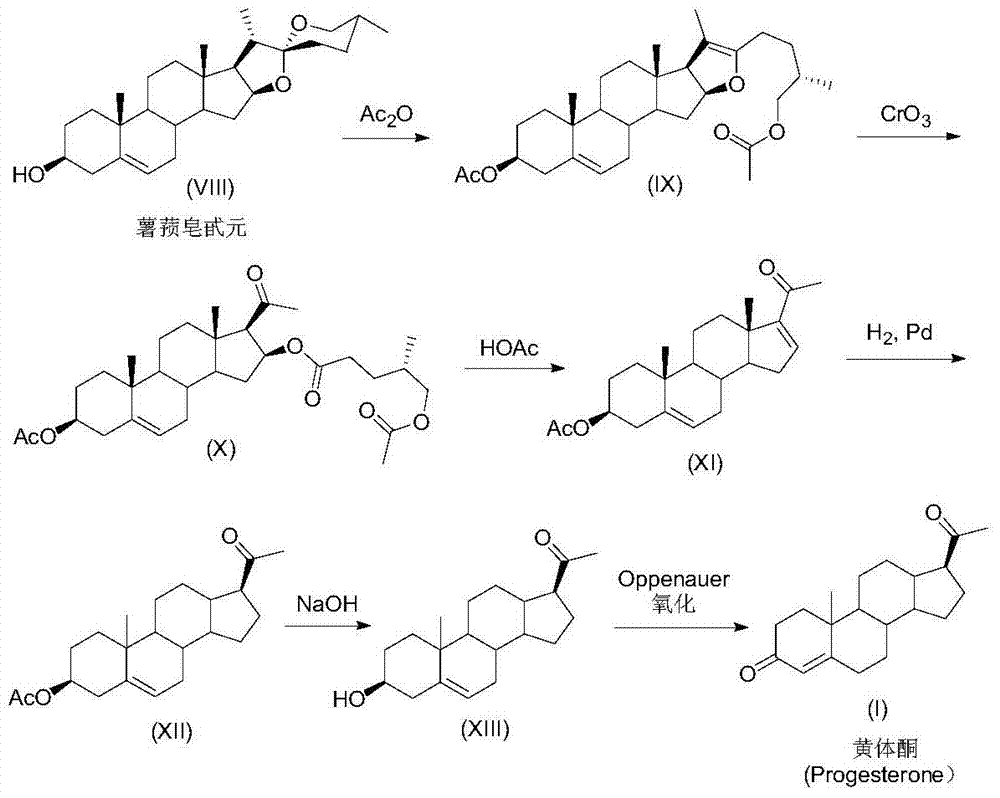
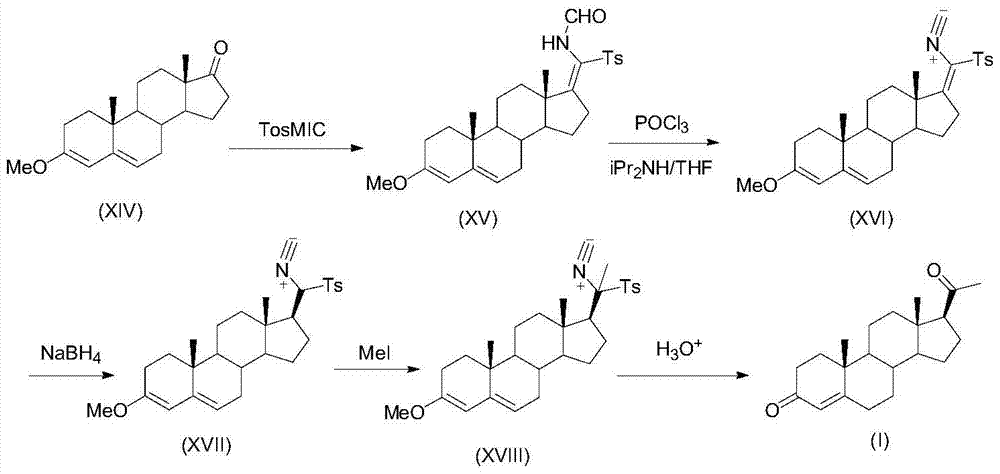
ActiveCN105481924AEasy to manufactureStable physical and chemical propertiesSteroidsIsocyanide compoundProgesterones
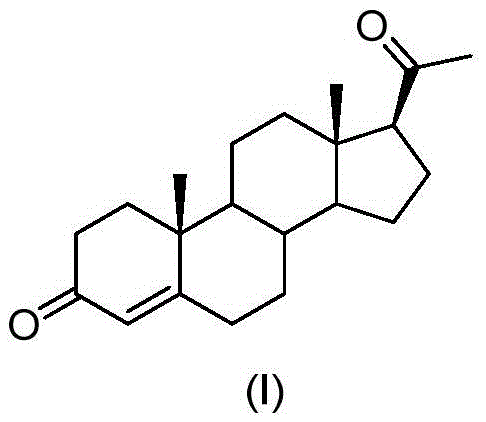
The invention discloses a preparation method for progesterone. The method comprises the following steps: with the compound 3-(2,2-dimethyl-1,3-dioxopropyl)-5-en-17-one (II) as a starting raw material, subjecting the starting raw material and a sulfonylmethyl isocyanide compound (III) to a condensation reaction under the action of alkali so as to prepare a formamido-sulfonylmethylene compound (IV); subjecting the compound (IV) to dehydration so as to obtain an isocyano-sulfonylmethylene compound (V); carrying out a reduction reaction on the compound (V) so as to prepare an isocyano-sulfonylmethyl compound (VI); subjecting the compound (VI) to methylation so as to obtain an isocyano-sulfonylethyl compound (VII); and carrying out hydrolysis on the compound (VII) so as to obtain the target products progesterone (I). The preparation method is simple in integral process, easy to operate, low in cost and suitable for large-scale production.
Owner:ZHEJIANG ZHUJI UNITED CHEM
Functional organic porous polymer as well as preparation method and application thereof
ActiveCN112724373AEasy to prepareFast preparation methodOther chemical processesPtru catalystOrganic solvent
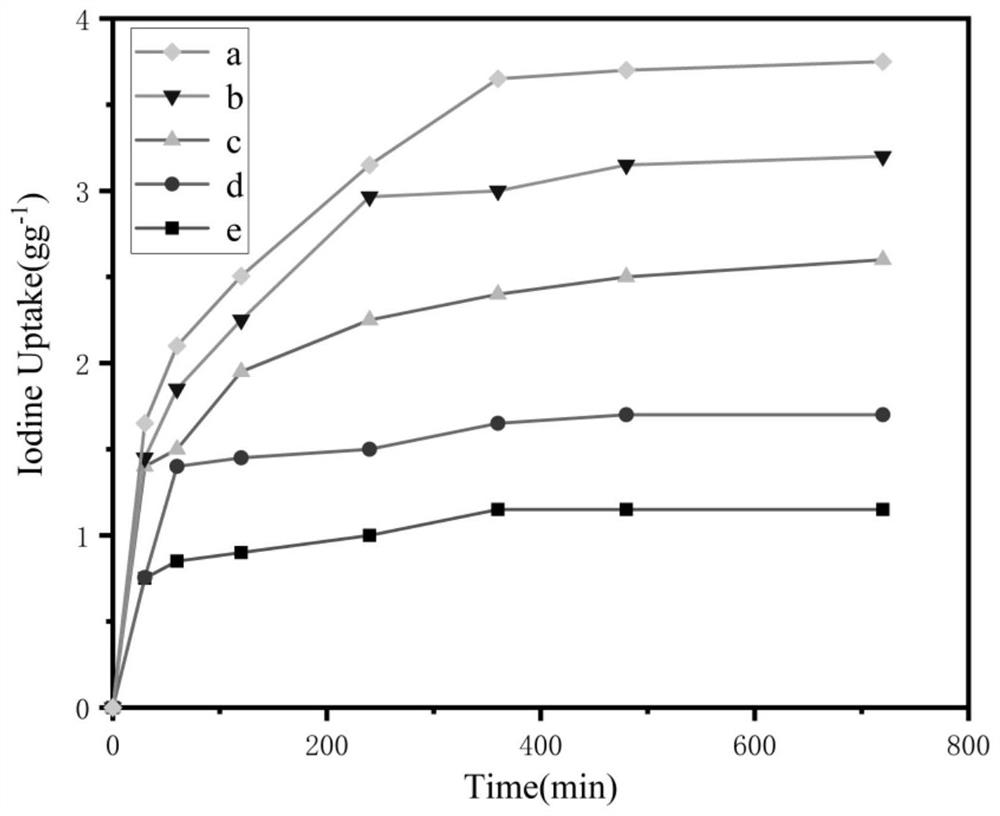
The invention relates to a functional organic porous polymer as well as a preparation method and application thereof. According to the preparation method, at least a dihalogenated aromatic compound, an aromatic compound containing at least two terminal alkynyl groups and an isocyanide compound are used as raw materials, and under the conditions of a catalyst and an acid absorbent, the functional organic porous polymer is prepared in an organic solvent through a one-pot method. The preparation method of the functional organic porous polymer is simple and rapid, the functional organic porous polymer can be obtained through one-step synthesis, experiment steps are simplified, and efficiency is improved. The functional organic porous polymer provided by the invention has excellent porous performance and high iodine adsorption capacity, has higher iodine adsorption performance than organic porous polymers without imine units, and is expected to be used as an iodine adsorption material for removing radioactive iodine in nuclear industry wastes.
Owner:SHANDONG UNIV
Method for synthesis of alpha-sulfonamido amide, carboxylic acid and hydroxamic acid derivatives
InactiveUS20030013910A1Organic compound preparationOrganic compound librariesCyanide compoundHydroxamic acid
A method of synthesizing alpha-sulfonamido amide, carboxylic acid or hydroxamic acid derivatives comprising providing a set of polymer-bound reactant(s) (sulfonamide, aldehyde or ketone, isocyanide or acid) to react with three sets of the other three reactants to form an array of polymer-bound alpha-sulfonamido amide-type intermediates and use of such intermediates for the preparation of combinatorial libraries.
Owner:ADVANCED SYNTECH
Non-aqueous electrolytes for lithium-ion batteries comprising an isocyanide
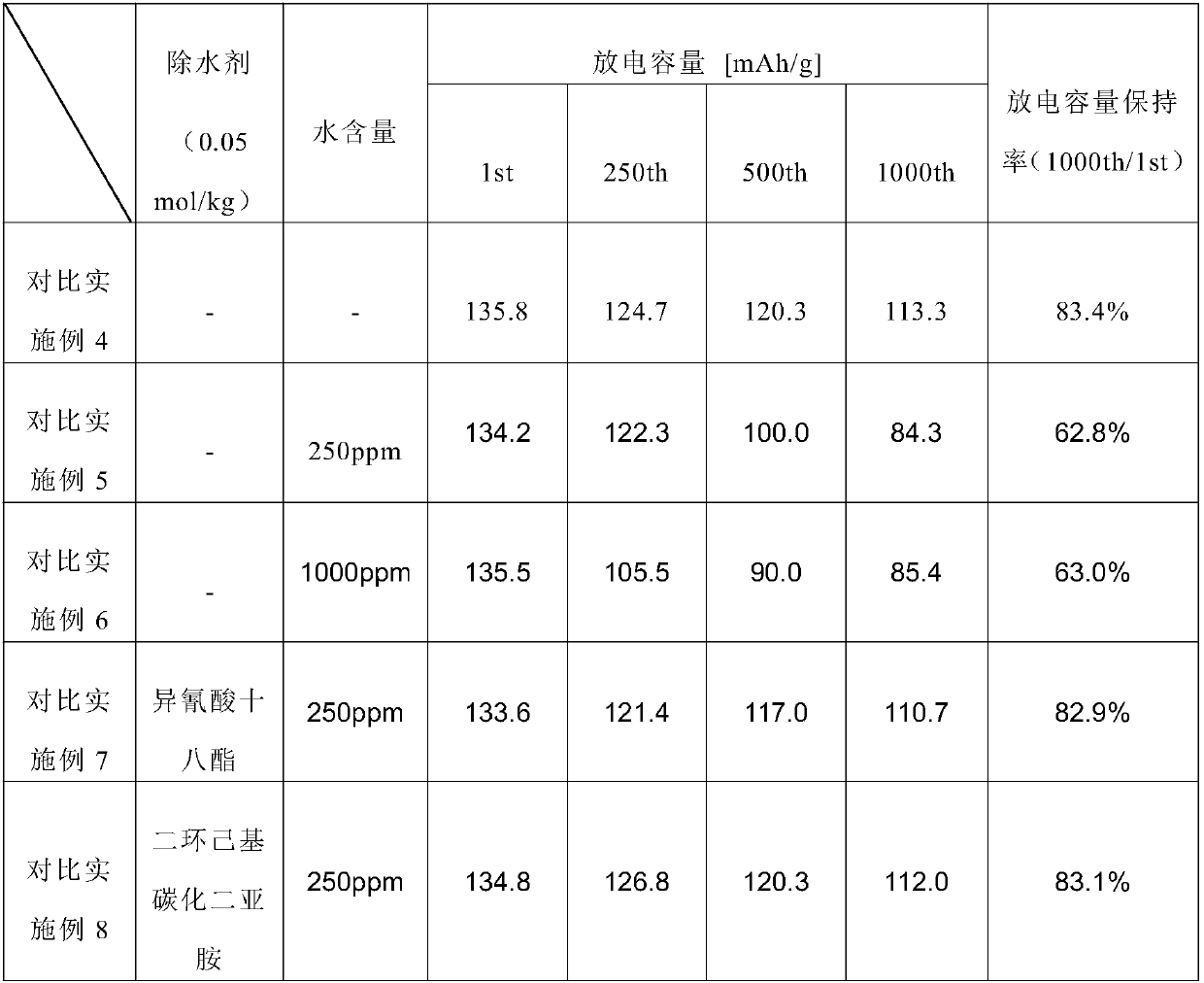
ActiveCN108028417AGood water removal performanceReduce water contentGroup 5/15 element organic compoundsCell electrodesLithium electrodeNon aqueous electrolytes
A nonaqueous electrolyte composition containing at least one organic isocyanide of formula (I) R-NC.
Owner:GOTION INC
Hydrosilylation reaction catalyst
ActiveUS10005797B2Easy to handleImprove responseSilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCyanide compoundPtru catalyst
A hydrosilylation reaction catalyst prepared from: a catalyst precursor comprising a transition metal compound, excluding platinum, belonging to group 8-10 of the periodic table, e.g., iron acetate, cobalt acetate, nickel acetate, etc.; and a ligand comprising an isocyanide compound such as t-butyl isocyanide. The hydrosilylation reaction catalyst has excellent handling and storage properties. As a result of using this catalyst, a hydrosilylation reaction can be promoted under gentle conditions.
Owner:KYUSHU UNIV +1
Alpha-aminoamidine polymers and uses thereof
α-Aminoamidine polymers and methods of preparing a-aminoamidine polymers by reacting by reacting one or more amines with one or more isocyanides and one or more aldehydes are described. Methods of preparing a-aminoamidine polymers from commercially available starting materials are also provided, wherein the starting materials are racemic or stereochemically pure. a-Aminoamidine polymers or salt forms thereof are preferably biodegradable and biocompatible and may be used in a variety of drug delivery systems and for other purposes as well such as, for example, coatings, additives, excipients, plastics, and materials, etc. Given the amino moiety of these α-aminoamidine polymers, they are particularly suited for the delivery of polynucleotides. Complexes, micelles, liposomes or particles containing the inventive α-aminoamidine polymers and polynucleotides can be prepared. The inventive α-aminoamidine polymers may also be used in preparing microparticles for drug delivery. They are particularly useful in delivering labile agents given their ability to buffer the pH of their surroundings.
Owner:MASSACHUSETTS INST OF TECH
Organopolysiloxane composition
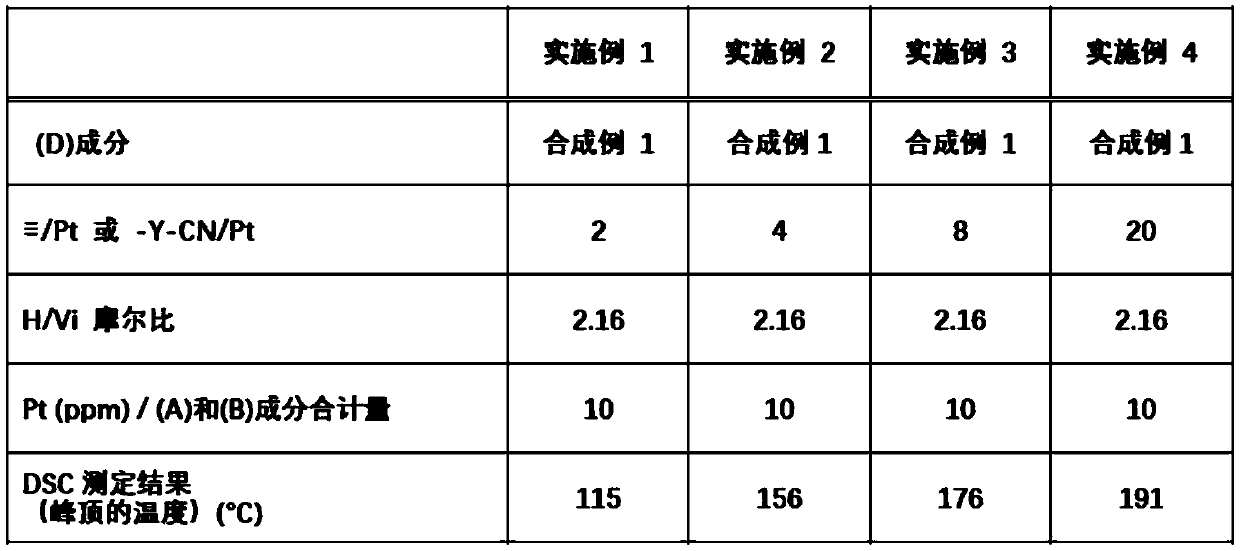
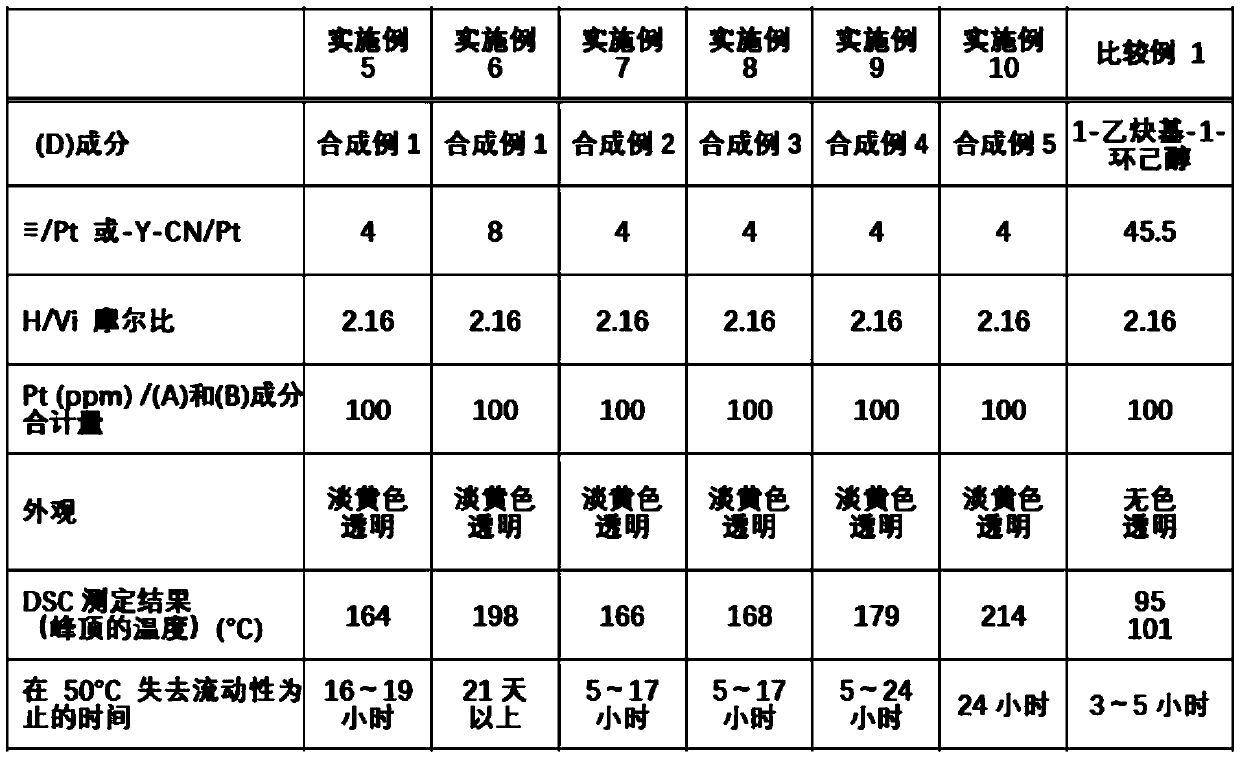
Provided is an organopolysiloxane composition comprising (A) an organopolysiloxane having at least two silicon atom-bonded alkenyl groups in each molecule, (B) an organohydrogenpolysiloxane having atleast two silicon atom-bonded hydrogen atoms in each molecule, (C) a platinum catalyst and (D) a specific isocyanide compound.
Owner:SHIN ETSU CHEM CO LTD
Hydrosilylation reaction catalyst
PendingUS20180200703A1Easy to handleHigh activitySilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsCyanide compoundPtru catalyst
A hydrosilylation reaction catalyst prepared from: a prescribed transition metal compound such as iron pivalate, cobalt pivalate, iron acetate, cobalt acetate, or nickel acetate; a ligand comprising t-butylisocyanide or another isocyanide compound; and a borane compound, Grignard reagent, alkoxysilane, or other prescribed promoter makes it possible to promote a hydrosilylation reaction under moderate conditions, and has exceptional handling properties and storage stability.
Owner:KYUSHU UNIV +1
A kind of preparation method of progesterone
ActiveCN105481924BEasy to manufactureStable physical and chemical propertiesSteroidsIsocyanide compoundProgesterones
The invention discloses a preparation method for progesterone. The method comprises the following steps: with the compound 3-(2,2-dimethyl-1,3-dioxopropyl)-5-en-17-one (II) as a starting raw material, subjecting the starting raw material and a sulfonylmethyl isocyanide compound (III) to a condensation reaction under the action of alkali so as to prepare a formamido-sulfonylmethylene compound (IV); subjecting the compound (IV) to dehydration so as to obtain an isocyano-sulfonylmethylene compound (V); carrying out a reduction reaction on the compound (V) so as to prepare an isocyano-sulfonylmethyl compound (VI); subjecting the compound (VI) to methylation so as to obtain an isocyano-sulfonylethyl compound (VII); and carrying out hydrolysis on the compound (VII) so as to obtain the target products progesterone (I). The preparation method is simple in integral process, easy to operate, low in cost and suitable for large-scale production.
Owner:ZHEJIANG ZHUJI UNITED CHEM
A kind of benzoxazepine antibacterial agent and its synthetic method
ActiveCN106831635BEnhanced inhibitory effectReduce manufacturing costBiocideOrganic chemistrySynthesis methodsStructural formula
The invention relates to benzoxazepin bacteriostats and a preparation method thereof. The chemical structural formula is shown in the specification, wherein substituents R1, R2 and R3 are chloro, bromo, methyl, normal-butyl, p-chlorophenyl, methyl acetate groups, tertiary butyl or cyclohexyl, and positions, the number and conjugate positions of the substituents are not fixed. According to the synthetic method, ortho-azidobenzoic acid compounds and aryl ketoaldehyde compounds and in-situ formed isocyanide compounds are subjected toa Passerini reaction, a formed product and triphenylphosphine are subjected toa Staudinger reaction, and polysubstituted benzoxazepin heterocyclic compounds and other important heterocyclic compounds are produced through ring closing of an intramolecular aza-Wittig reaction. The key point of the invention is to provide one novel synthetic method which adopts fewer steps and has high yield to synthesize the novel benzoxazepin compounds. The compounds have better inhibitory activity on Rhizoctonia solani, Penicillium digitatum and Penicillium italicum and can be used as the bacteriostats.
Owner:CHINA THREE GORGES UNIV
Organopolysiloxane composition
Provided is an organopolysiloxane composition, comprising: (A) an organopolysiloxane having at least two silicon atom-bonded alkenyl groups in each molecule, (B) an organohydrogenpolysiloxane having at least two silicon atom-bonded hydrogen atoms in each molecule, (C) a platinum catalyst, and (D) a specific isocyanide compound.
Owner:SHIN ETSU CHEM IND CO LTD
Resin composition containing supported platinum catalyst, thermosetting organopolysiloxane composition using the resin composition, and method for curing the thermosetting organopolysiloxane composition
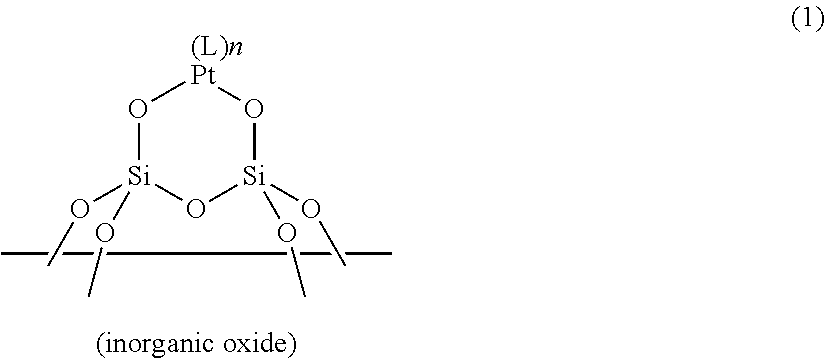
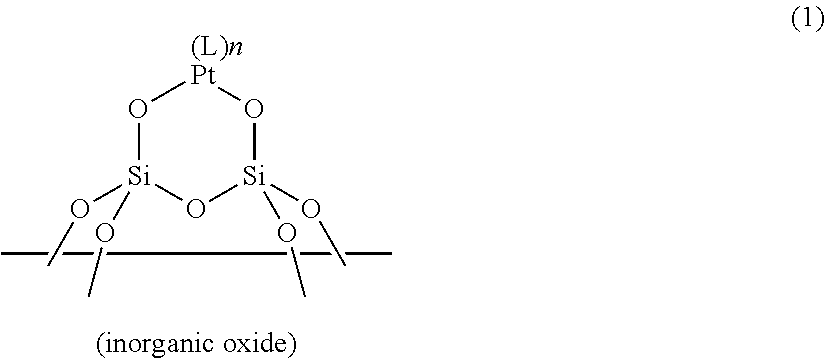
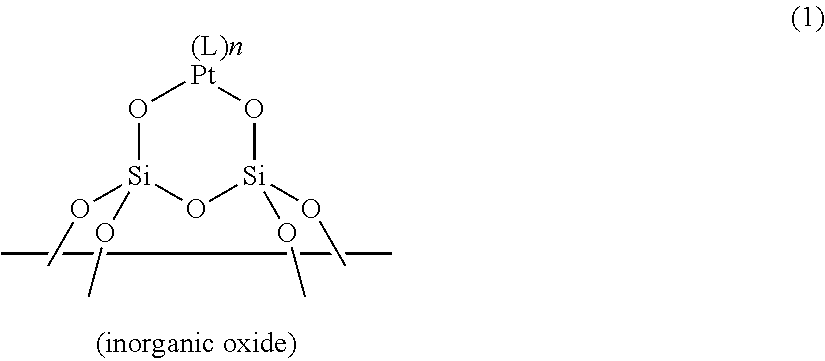
ActiveUS20190100649A1Sufficient quick curabilitySufficient storabilityCatalyst protectionOrganic-compounds/hydrides/coordination-complexes catalystsPlatinum complexIsocyanide compound
A resin composition includes: (a) a supported platinum catalyst having a structure shown by the following general formula (1) in which a platinum complex is supported on a surface of an inorganic oxide; and (b) a thermoplastic matrix resin. The resin composition is usable as an addition-reaction catalyst capable of imparting sufficient storability and quick curability to an addition-reaction curable composition.In the formula, L represents a ligand selected from carbon monoxide, an olefin compound, an amine compound, a phosphine compound, an N-heterocyclic carbene compound, a nitrile compound, and an isocyanide compound; and n represents the number of Ls and an integer from 0 to 2.
Owner:SHIN ETSU CHEM IND CO LTD +1
Cyclic amino acid molecules and methods of preparing the same
ActiveUS9260479B2Silicon organic compoundsPeptide preparation methodsCyclic Amino AcidsOrganic chemistry
Owner:THE GOVERNING COUNCIL OF THE UNIV OF TORONTO
Method for producing transition metal-isocyanide complex
ActiveUS11034712B2The fermentation process is simpleSimple methodRuthenium organic compoundsRhodium organic compoundsCyanide compoundIsocyanide compound
Owner:KYUSHU UNIV +1
Preparation method of tetraboronic acid compounds, and tetraboronic acid compounds
ActiveUS10689404B1Increase contentDelayed reaction timeGroup 3/13 element organic compoundsCyanide compoundIsocyanide compound
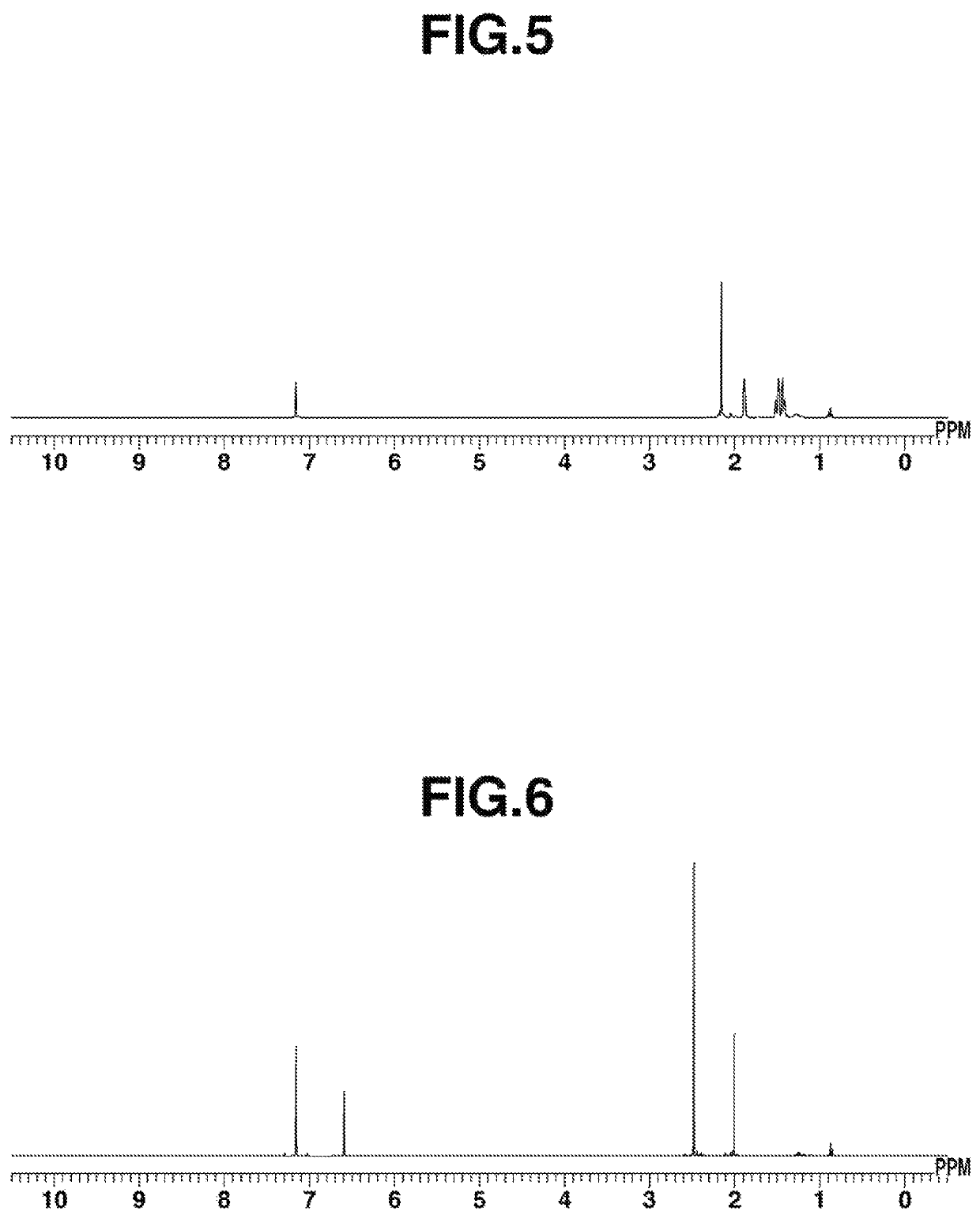
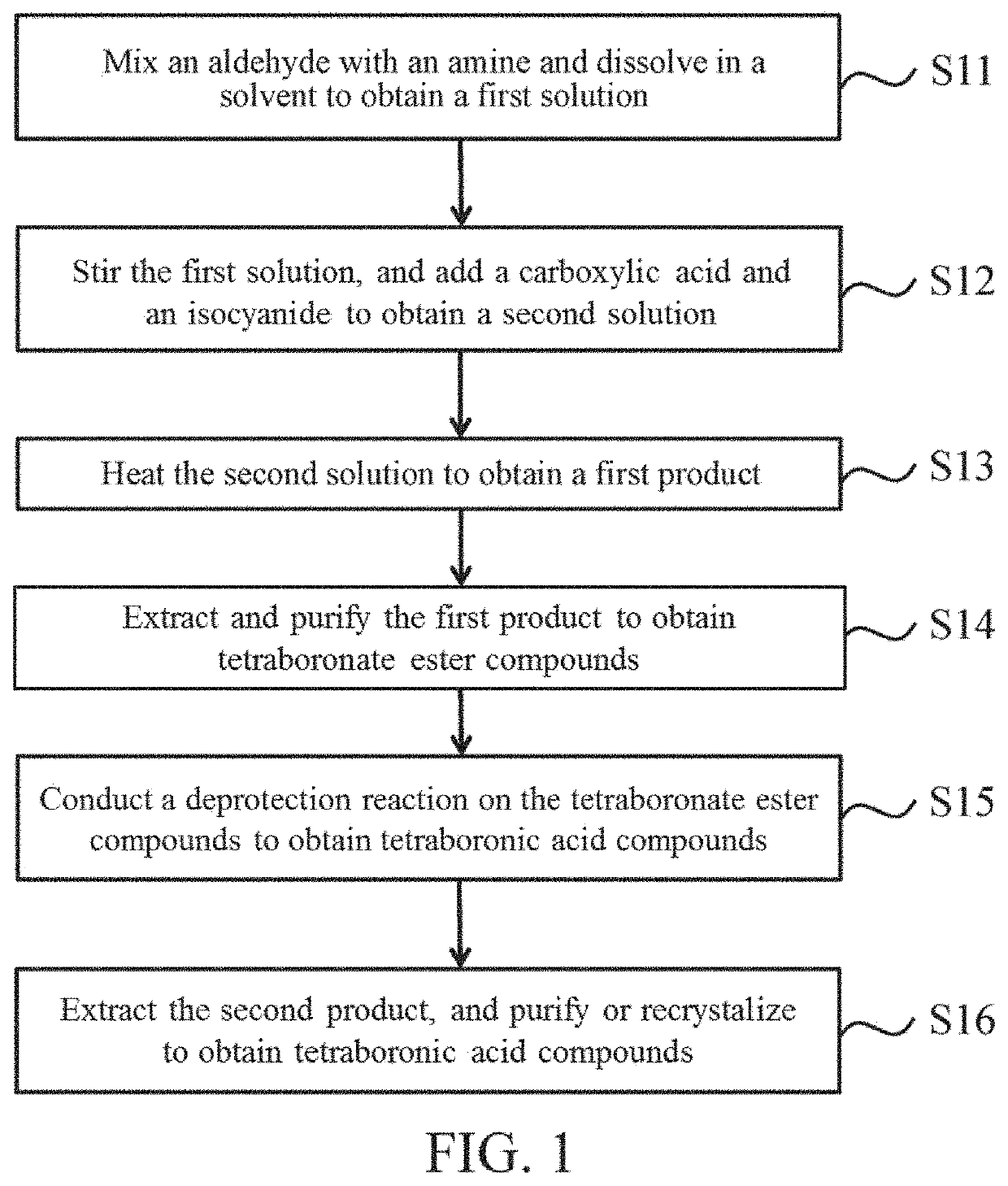
A preparation method of tetraboronic acid compounds includes mixing aldehydes with amines and dissolving them in a solvent to obtain a first solution, stirring the first solution, adding carboxylic acid and isocyanide to obtain a second solution, heating the second solution to obtain a first product, extracting and purifying the first product to obtain tetraboronate ester compounds, and executing a deprotection reaction on the tetraboronate ester compounds under heating by microwave to obtain a second product. The second product contains tetraboronic acid compounds, and the tetraboronic acid compounds have the structure as shown in formula (I).
Owner:TAMKANG UNIVERSITY
Method for synthesizing indolizine compound under catalysis of silver
ActiveCN113200980AEasy to separate and purifyEasy to operateOrganic chemistryChemical recyclingPtru catalystOrganic synthesis
The invention discloses a method for synthesizing indolizine compounds under the catalysis of silver, which comprises the following steps of in an organic solvent system, stirring N-benzoylmethyl pyridinium bromide as shown in a formula (1) and an isocyanide compound as shown in a formula (2) according to a feeding molar ratio of 1: (1.2-2.0) in the presence of a metal silver salt as a catalyst to react in the air under an alkaline condition, and carrying out TLC tracking detection until the reaction is complete, and carrying out post-treatment on the reaction liquid to obtain the indolizine compound as shown in a formula (3). The method is easy to operate, raw materials and reagents are easy to obtain, reaction conditions are mild, a reaction system is environmentally friendly, products are easy to separate and purify, the yield reaches up to 91%, and the method is suitable for efficient and high-yield preparation of indolizine compounds and particularly suitable for synthesis of various 1, 2-substituted indolizine compounds. The method is suitable for large-scale industrial production, and has wide application prospects and important significance in organic synthesis.
Owner:XUZHOU NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com