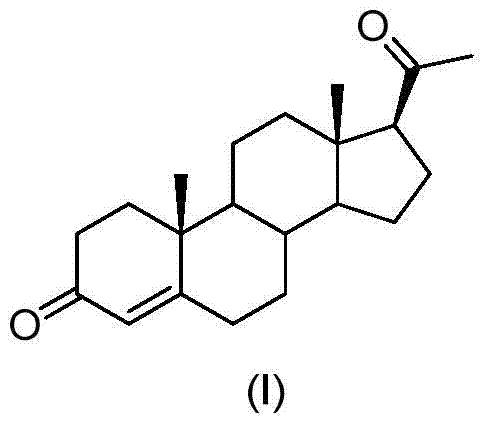
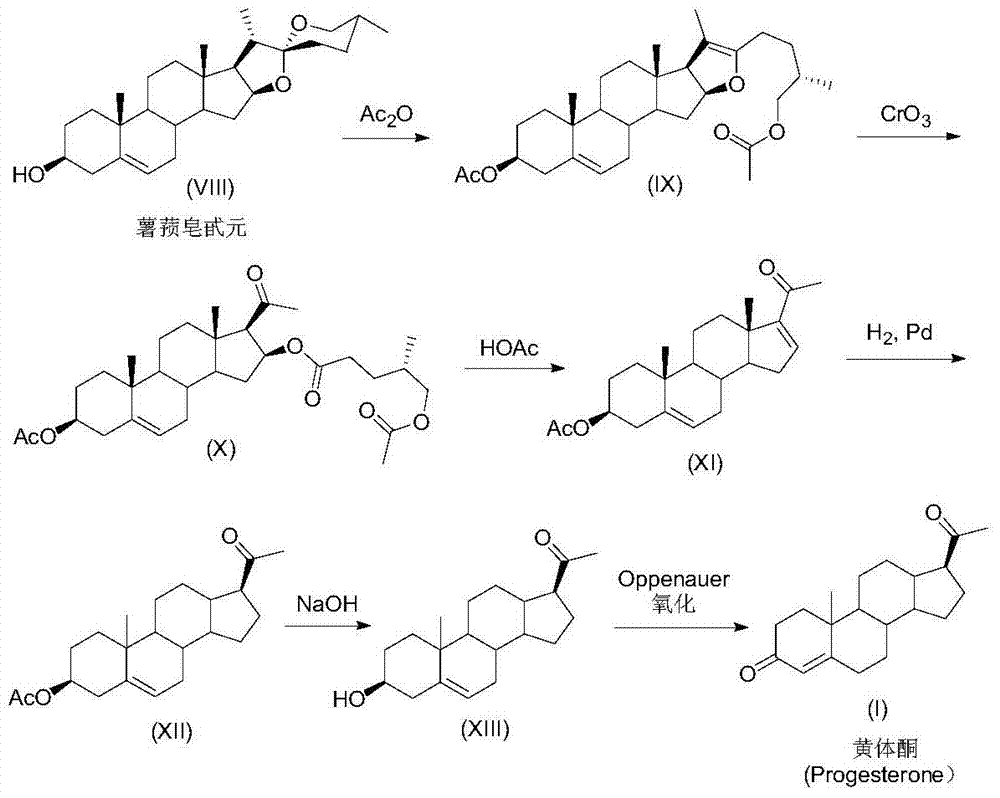
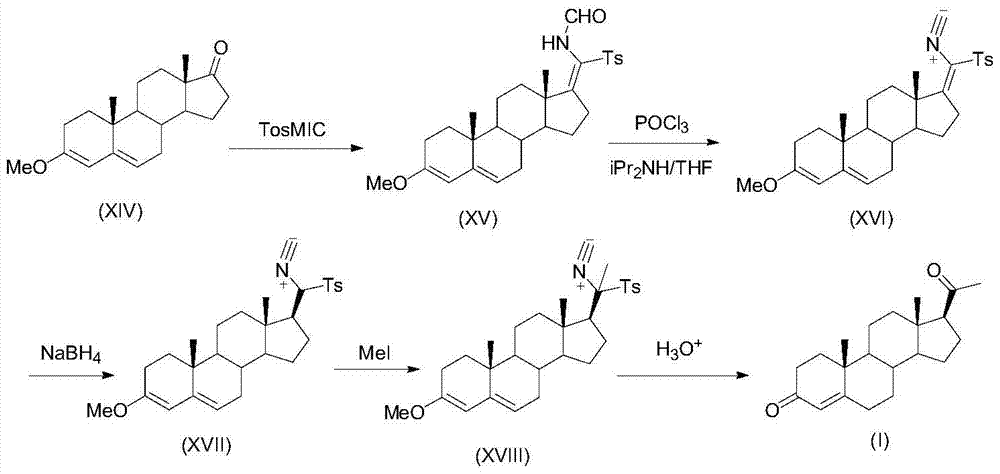
A kind of preparation method of progesterone
A progesterone and compound technology, applied in the field of preparation of organic steroids, can solve the problems of lack of competitiveness, environmental pollution, long production cycle, etc., and achieve the effect of simple process, environment-friendly and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: (E)-17-formamido-tosylmethylene-3-(2,2-dimethyl-1,3-dioxopropyl)androst-5-ene (Compound IV ) Preparation 1
[0030] Under a dry ice-acetone bath, THF (40mL), TosMIC (1.95g), compound (II) (3.73g), and potassium tert-butoxide (2.48g) were sequentially added to react for 2h. Extracted with methyl chloride, and the organic layer was dried and concentrated to obtain 5.4 g of compound (IV) as a pale yellow foamy solid.
Embodiment 2
[0031] Example 2: (E)-17-formamido-tosylmethylene-3-(2,2-dimethyl-1,3-dioxopropyl)androst-5-ene (Compound IV ) Preparation 2
[0032] Under the dry ice-acetone bath, add toluene (50mL), TosMIC (2.93g), compound (II) (3.73g), lithium tert-butoxide (1.77g) in sequence, react for 2h, pour the reaction solution into water, and use two Extracted with methyl chloride, and the organic layer was dried and concentrated to obtain 5.3 g of compound (IV) as a pale yellow foamy solid.
Embodiment 3
[0033] Example 3: (E)-17-isocyano-p-toluenesulfonylmethylene-3-(2,2-dimethyl-1,3-dioxopropyl)androst-5-ene (Compound V ) Preparation 1
[0034] Compound (IV) (6.38g) prepared above was dissolved in tetrahydrofuran (50mL), diisopropylamine (4.9mL) was added dropwise at -10°C, phosphorus oxychloride (1.1mL) was added dropwise, and reacted for 2h. The reaction solution was poured into 100 mL of ice water containing 5 g of sodium bicarbonate, filtered, washed with water, and dried to obtain 5.9 g of yellow solid compound (V).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com