N-heterocyclic condensed tryptamine-beta-lactam derivative as well as preparation method and application thereof
A technology for synthesizing tryptamine and its derivatives, which is applied in the field of N-heterocyclic fused tryptamine-β-lactam derivatives and its preparation, achieving the effects of mild reaction, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
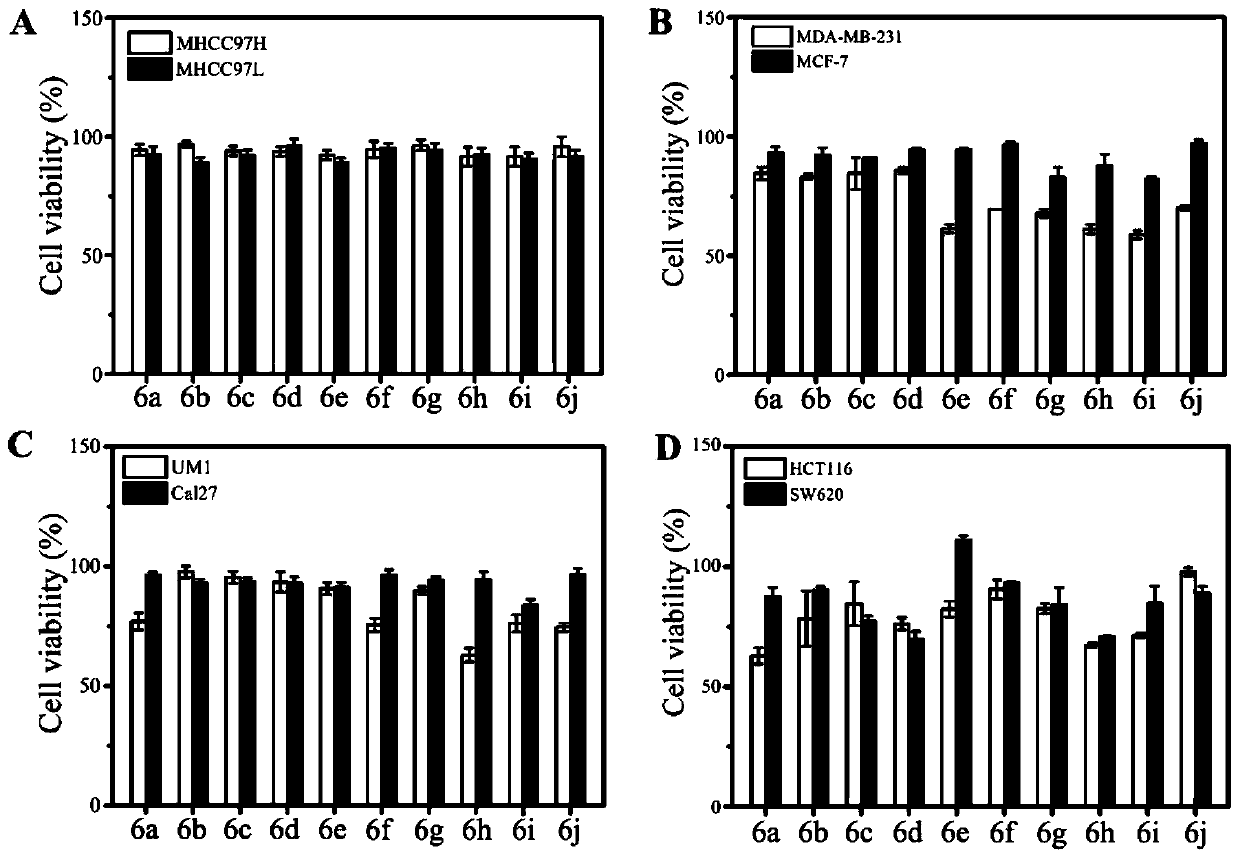
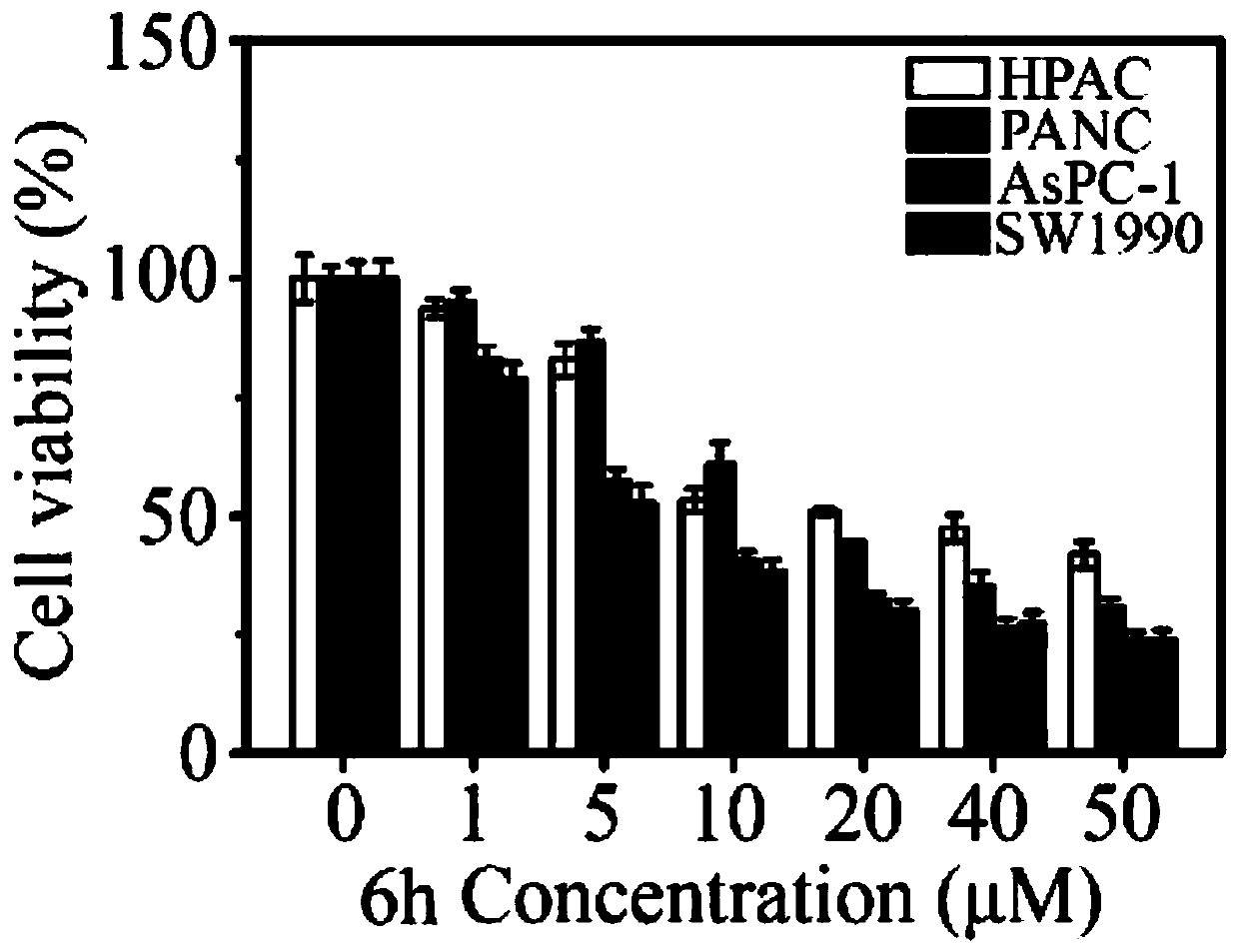
[0049] This example describes the N-heterocyclic fused tryptamine-β-lactam derivative 6a and its preparation method, R in compound 6a 1 is phenyl, R 2 For benzyl, the name of compound 6a is 1-(2-(1H-indol-3-yl)ethyl)-N-benzyl-3-methylene-4-oxo-2-phenylazepine Cyclobutane-2-carboxamide, its chemical structural formula is as follows:
[0050]
[0051] The preparation method of compound 6a is as follows:
[0052] At room temperature, add 1.00 mmol benzaldehyde and 1.00 mmol propiolic acid into a microwave bottle (10 mL) containing 2 mL methanol and stir for 5 minutes, then add 1.00 mmol tryptamine and 1.00 mmol benzyl isonitrile and stir the reaction overnight. The isonitrile was determined by layer chromatography to disappear, and the reaction was completed. The solvent methanol was removed under a gentle nitrogen flow, and the obtained residue was dissolved in 3.0 mL of acetonitrile, and 2.0 mmol K 2 CO 3 , sealed and placed in a microwave oven at 120 ° C for 10 min, coo...
Embodiment 2
[0055] This example describes the N-heterocyclic fused tryptamine-β-lactam derivative 6b and its preparation method, R in compound 6b 1 is 4-fluorophenyl, R 2 For benzyl, the name of compound 6b is 1-(2-(1H-indol-3-yl)ethyl)-N-benzyl-2-(4-fluorophenyl)-3-methylene-4 -Oxazetidine-2-carboxamide, its chemical structural formula is as follows:
[0056]
[0057] The preparation method of compound 6b is as follows:
[0058] At room temperature, 1.00 mmol of 4-fluorobenzaldehyde and 1.00 mmol of propiolic acid were added to a microwave bottle (10 mL) containing 2 mL of methanol and stirred for 5 minutes, followed by addition of 1.00 mmol of tryptamine and 1.00 mmol of benzyl isonitrile and stirred overnight, The disappearance of isonitrile was determined by thin-layer chromatography, and the reaction was completed. The solvent methanol was removed under a gentle nitrogen flow, and the obtained residue was dissolved in 3.0 mL of acetonitrile, and 2.0 mmol K 2 CO 3 , sealed and ...
Embodiment 3
[0061] This example describes the N-heterocyclic fused tryptamine-β-lactam derivative 6c and its preparation method, R in compound 6c 1 is 4-chlorophenyl, R 2 For benzyl, the name of compound 6c is 1-(2-(1H-indol-3-yl)ethyl)-N-benzyl-2-(4-chlorophenyl)-3-methylene-4 -Oxazetidine-2-carboxamide, its chemical structural formula is as follows:
[0062]
[0063] The preparation method of compound 6c is as follows:
[0064] At room temperature, 1.00 mmol of 4-chlorobenzaldehyde and 1.00 mmol of propiolic acid were added into a microwave bottle (10 mL) containing 2 mL of methanol and stirred for 5 minutes, followed by addition of 1.00 mmol of tryptamine and 1.00 mmol of benzyl isonitrile and stirred overnight, The disappearance of isonitrile was determined by thin-layer chromatography, and the reaction was completed. The solvent methanol was removed under a gentle nitrogen flow, and the obtained residue was dissolved in 3.0 mL of acetonitrile, and 2.0 mmol K 2 CO 3 , sealed an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com