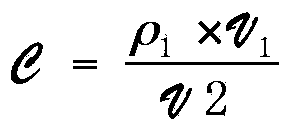Patents
Literature
88 results about "Metallic mercury" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metallic mercury is the pure form of mercury. It is a shiny, silver-white, odorless liquid much heavier than water that is used in thermometers, dental fillings and batteries and is also used in the production of chlorine gas and caustic soda.
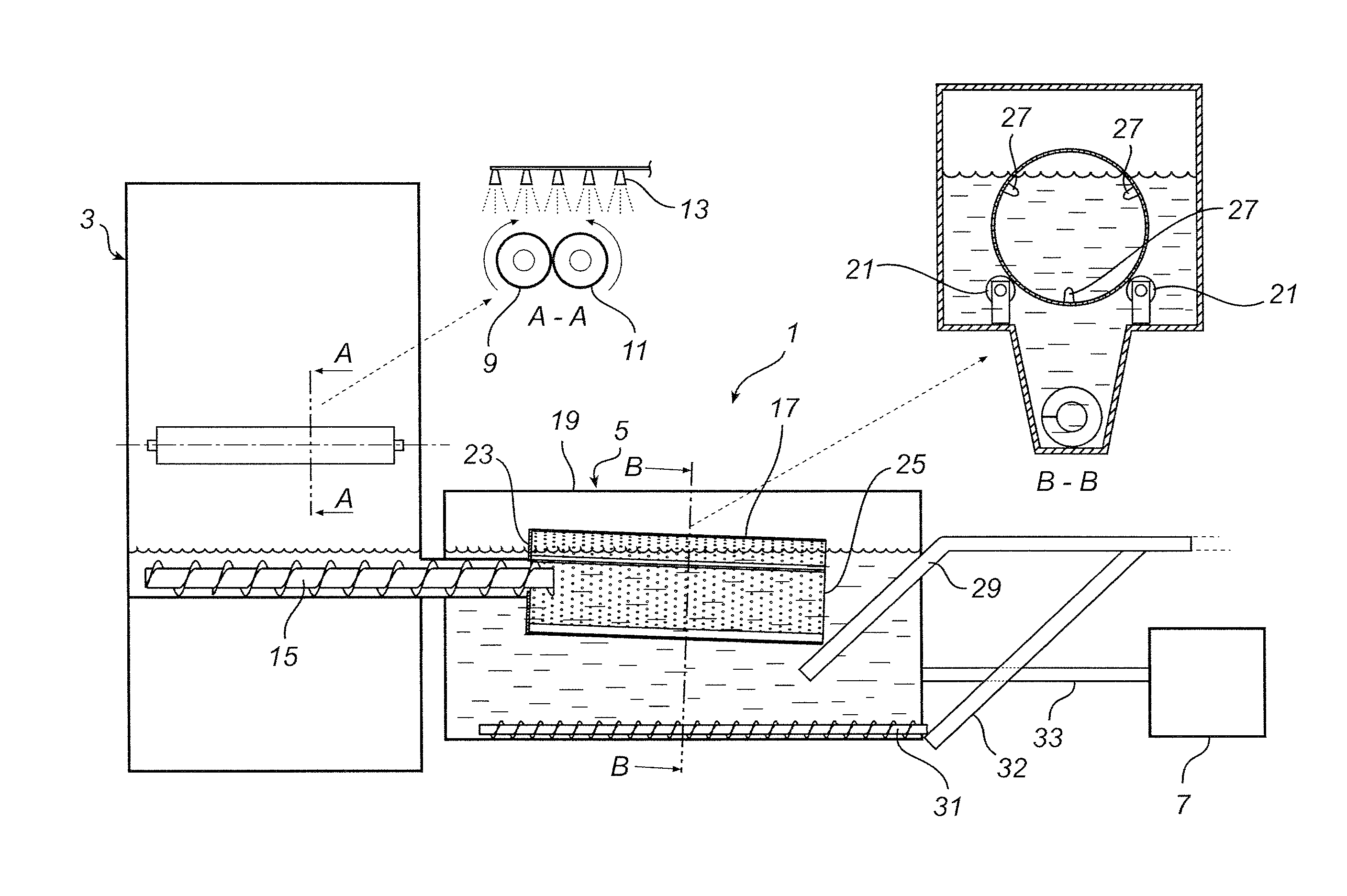
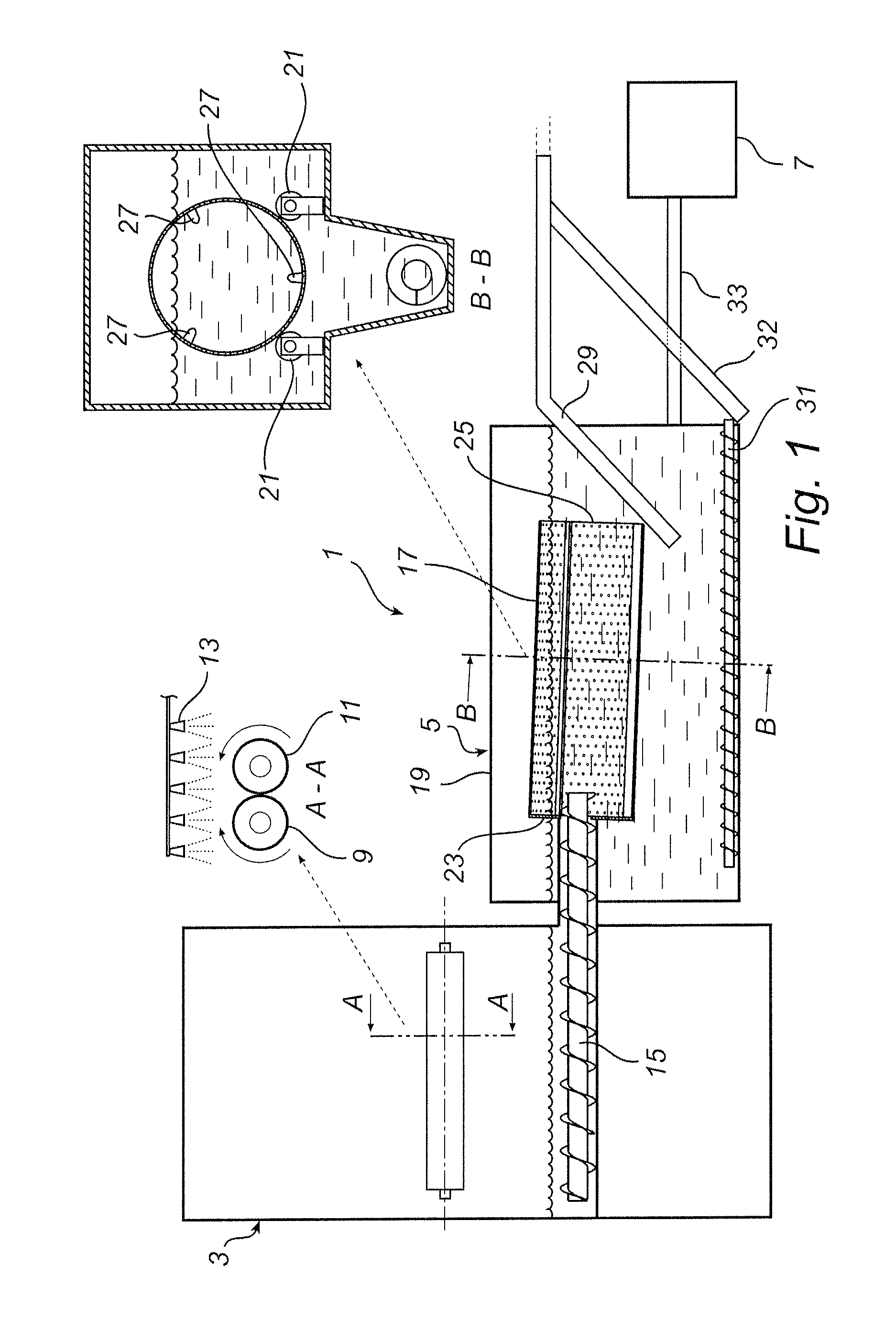
Method of removing mercury from flue gases
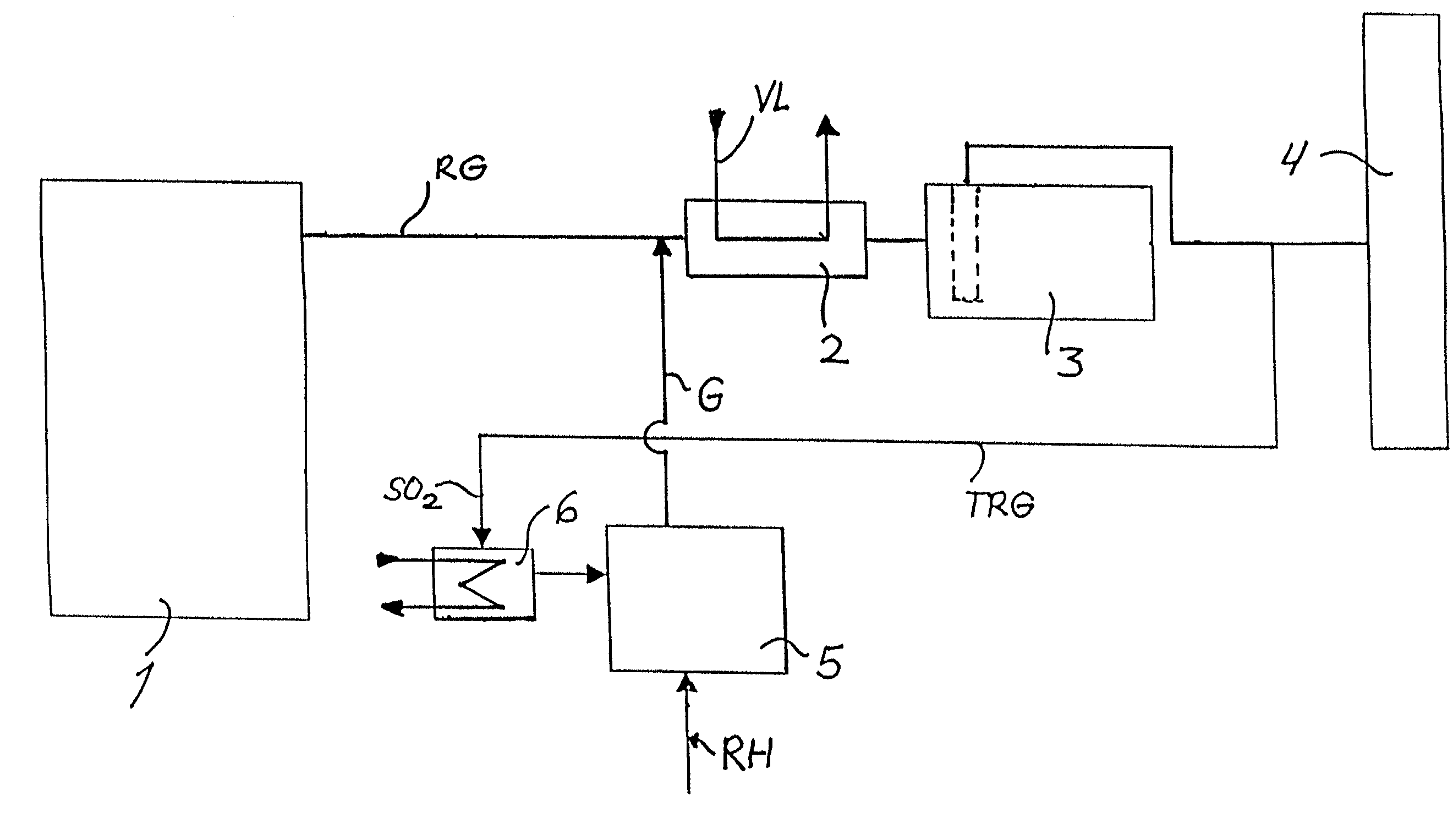
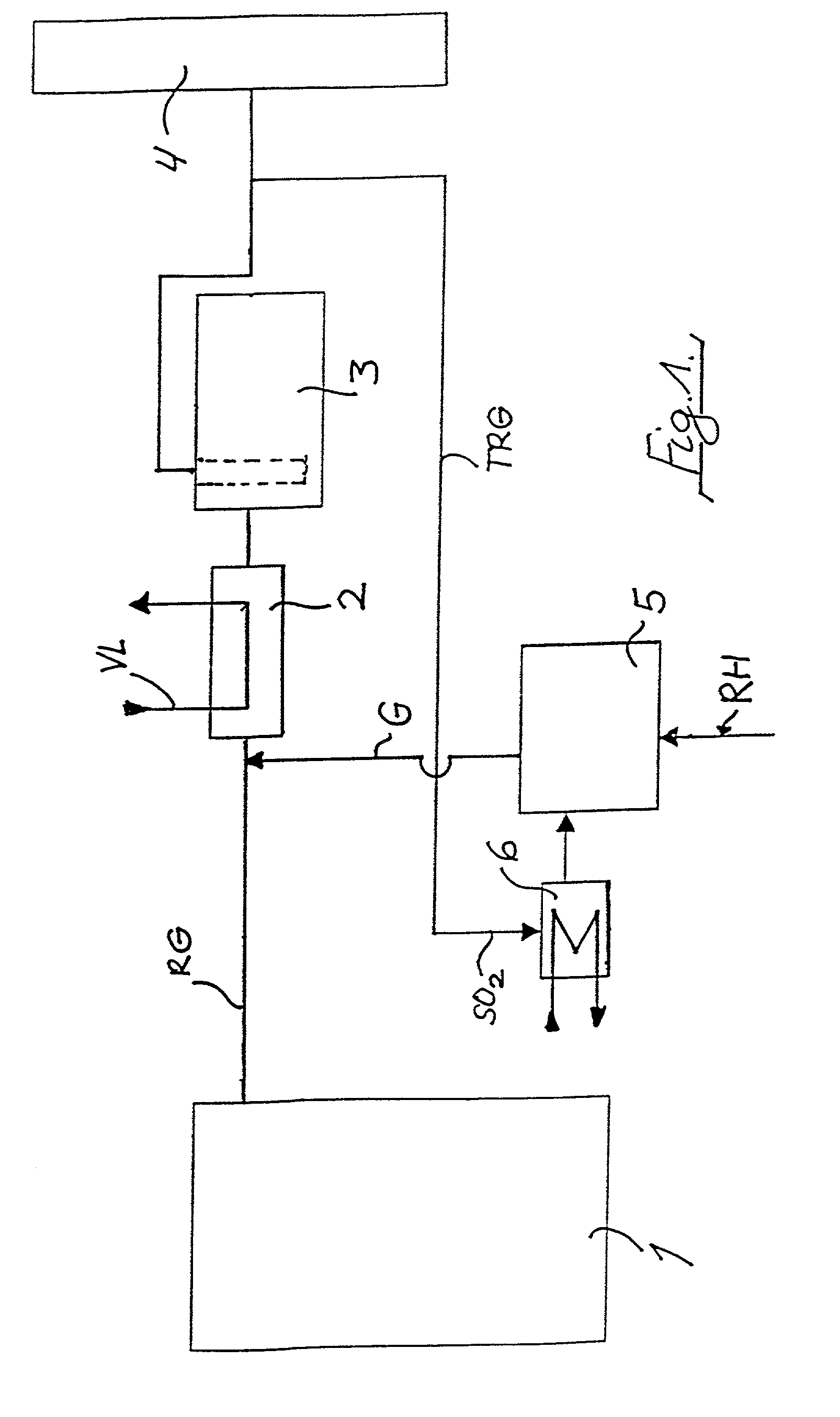
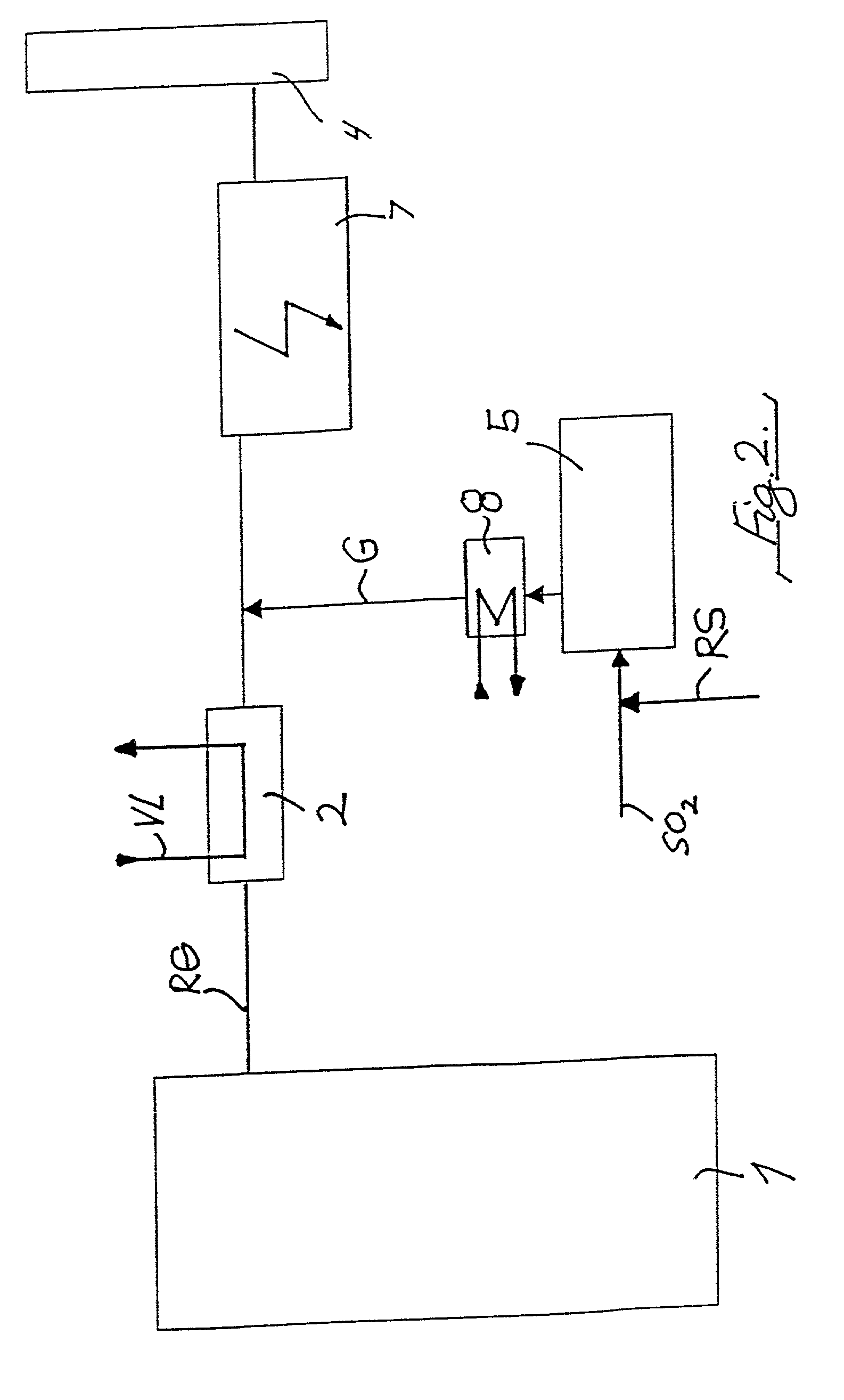
A method of removing metallic mercury and ionic mercury from flue gases, especially of a power plant, is provided. A gas that contains sulfur dioxide, or other adequate amounts of sulfur in the form of H2S or COS, and a gas that contains hydrogen, are conveyed to a catalyzer for producing a gas that contains elemental sulfur and hydrogen sulfide. This gas is conveyed to flue gas upstream of a separator, wherein mercury in the flue gas reacts with the sulfur and ionic sulfur in the gas and is separated out in the separator.
Owner:FISIA DEUT GMBH AND +1
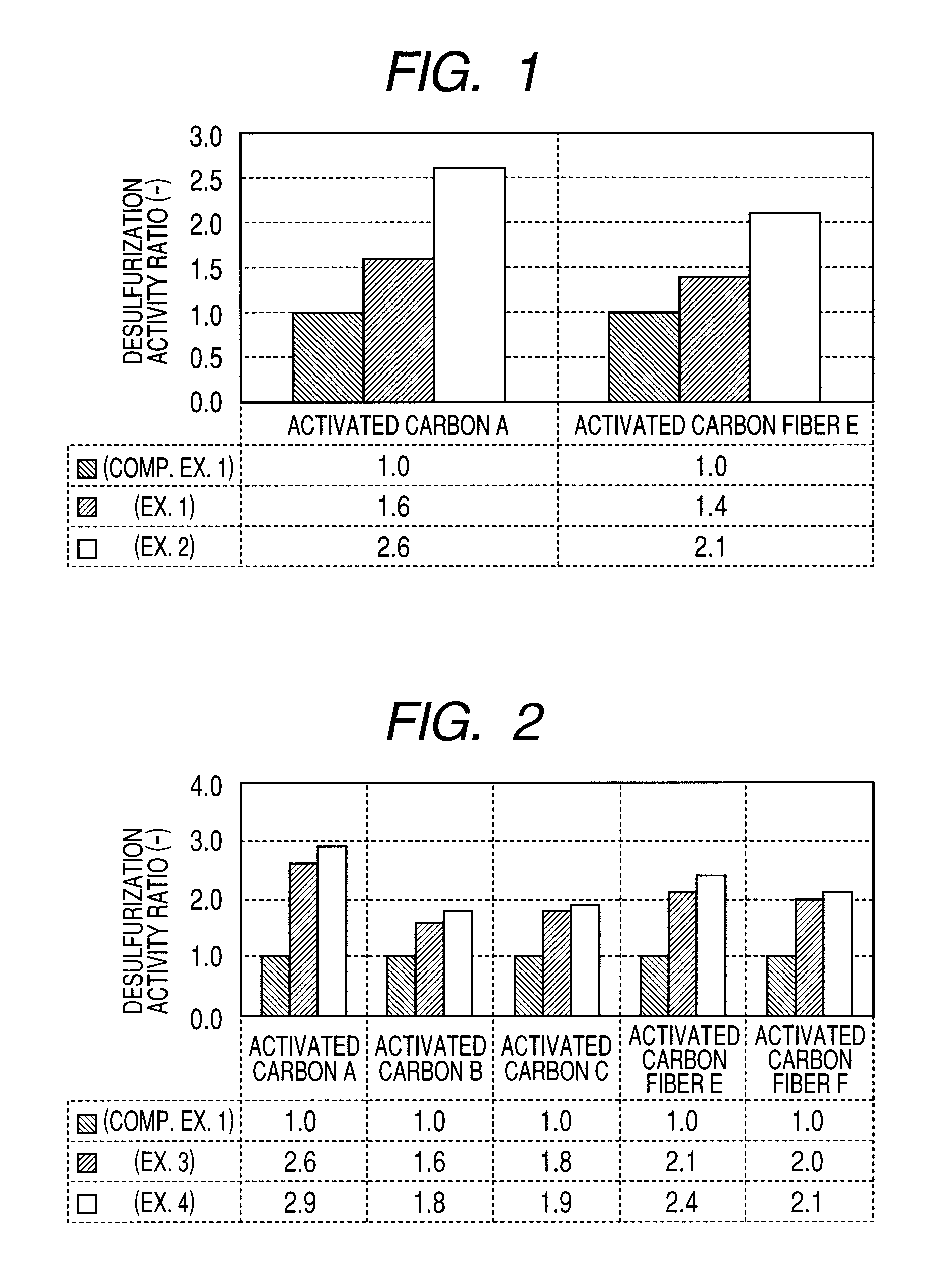
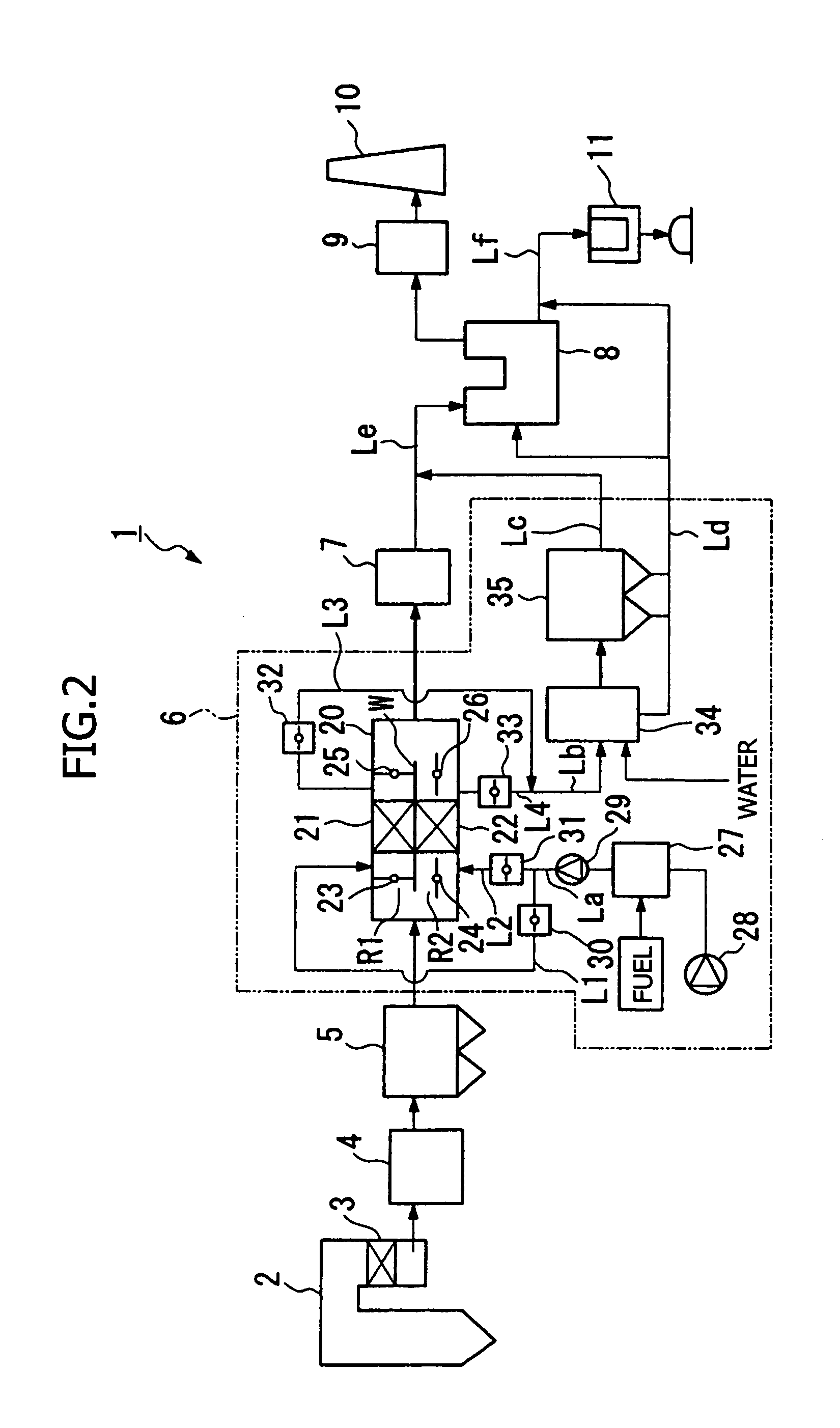
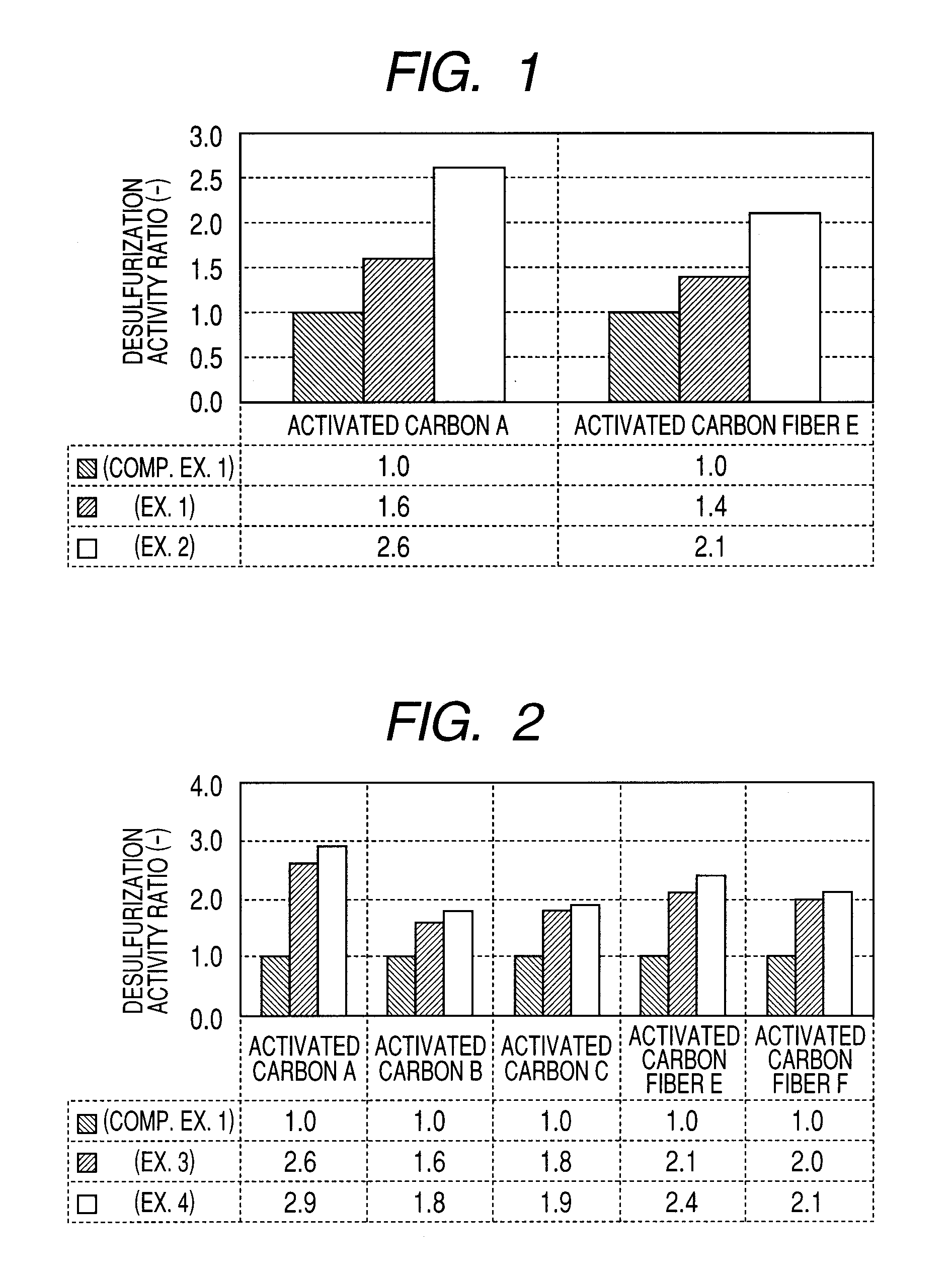
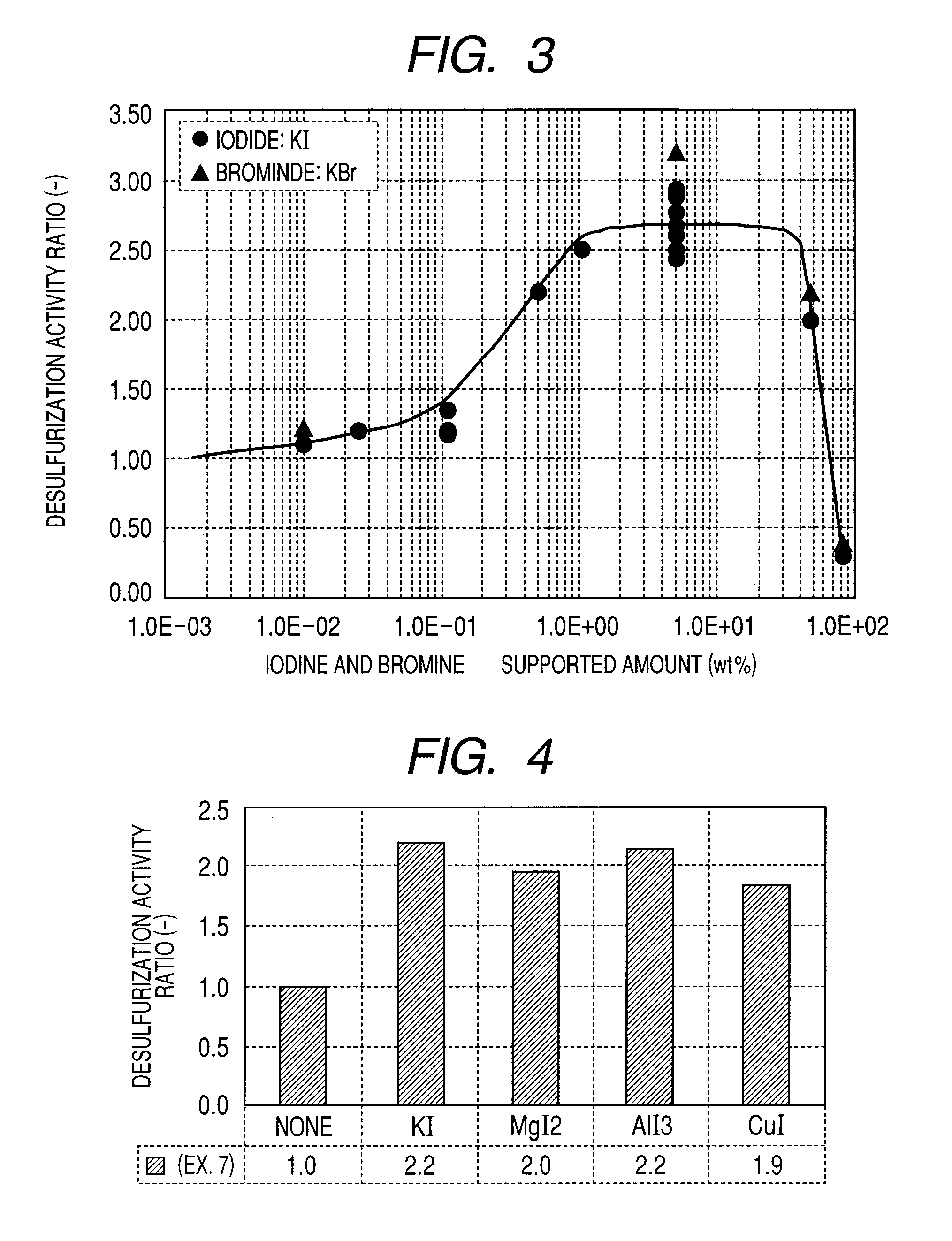
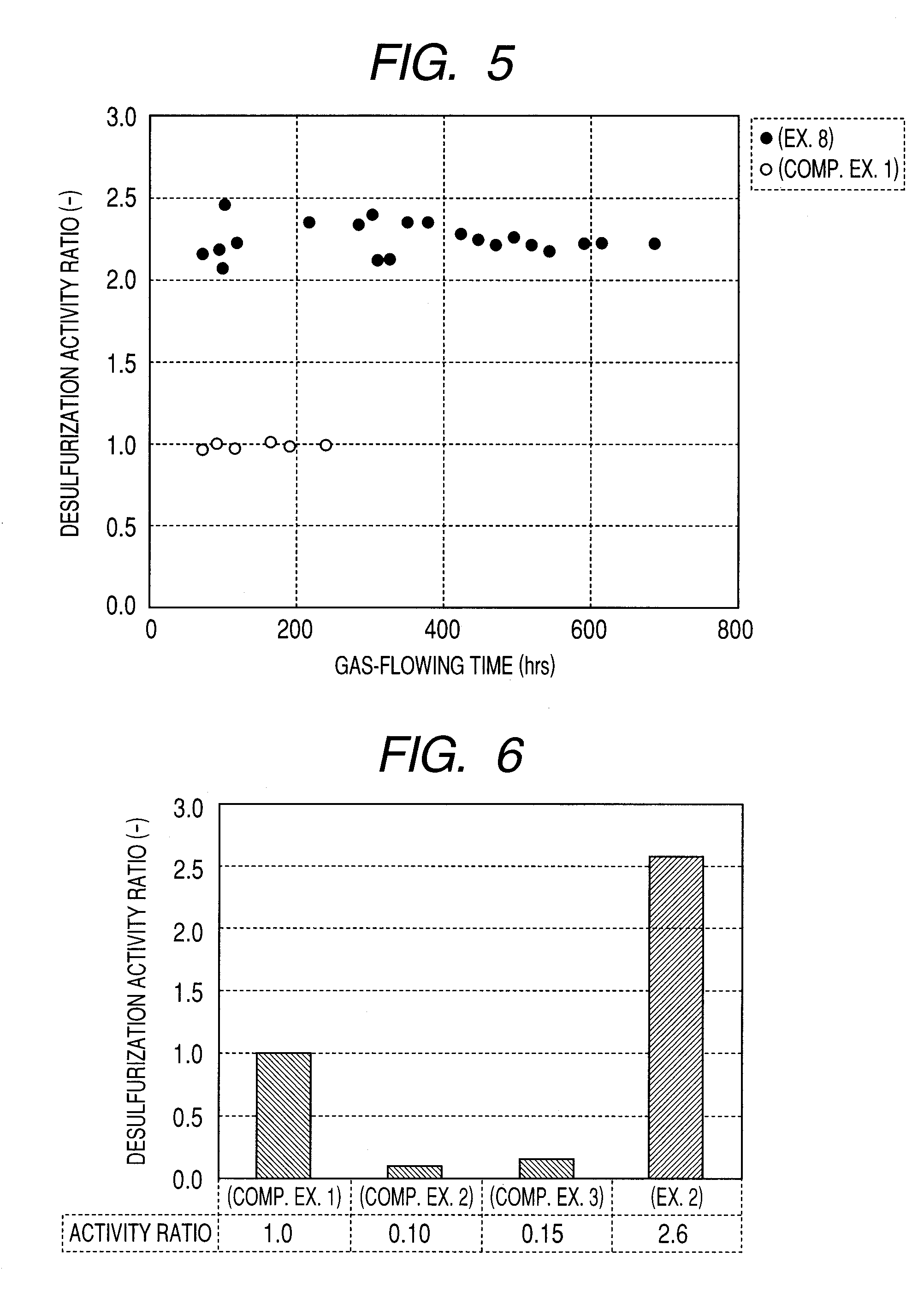
Carbon-based catalyst for flue gas desulfurization and method of producing the same and use thereof for removing mercury in flue gas
ActiveUS8524186B2Improve desulfurization performanceReduce the amount requiredGas treatmentOrganic-compounds/hydrides/coordination-complexes catalystsWater vaporSorbent
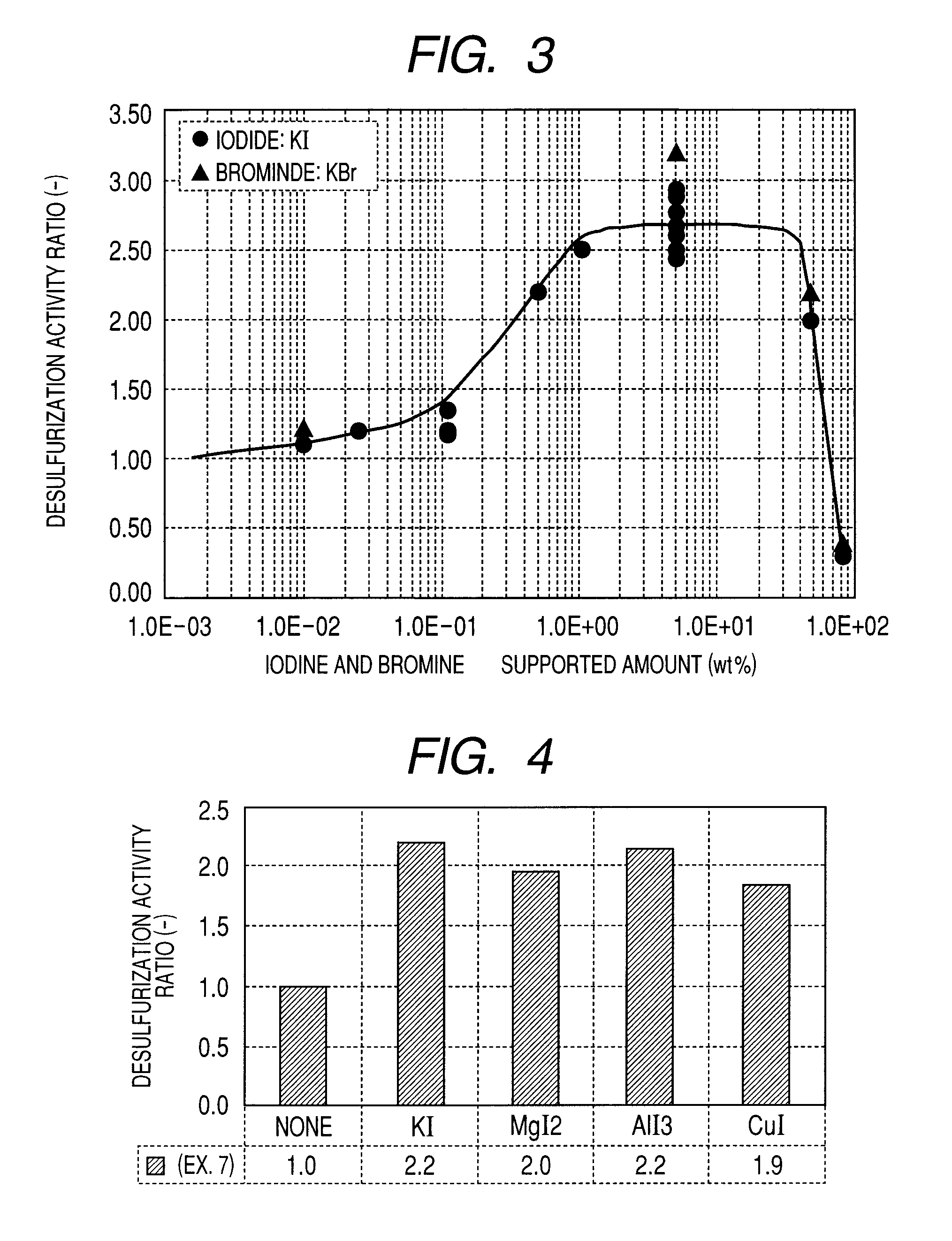
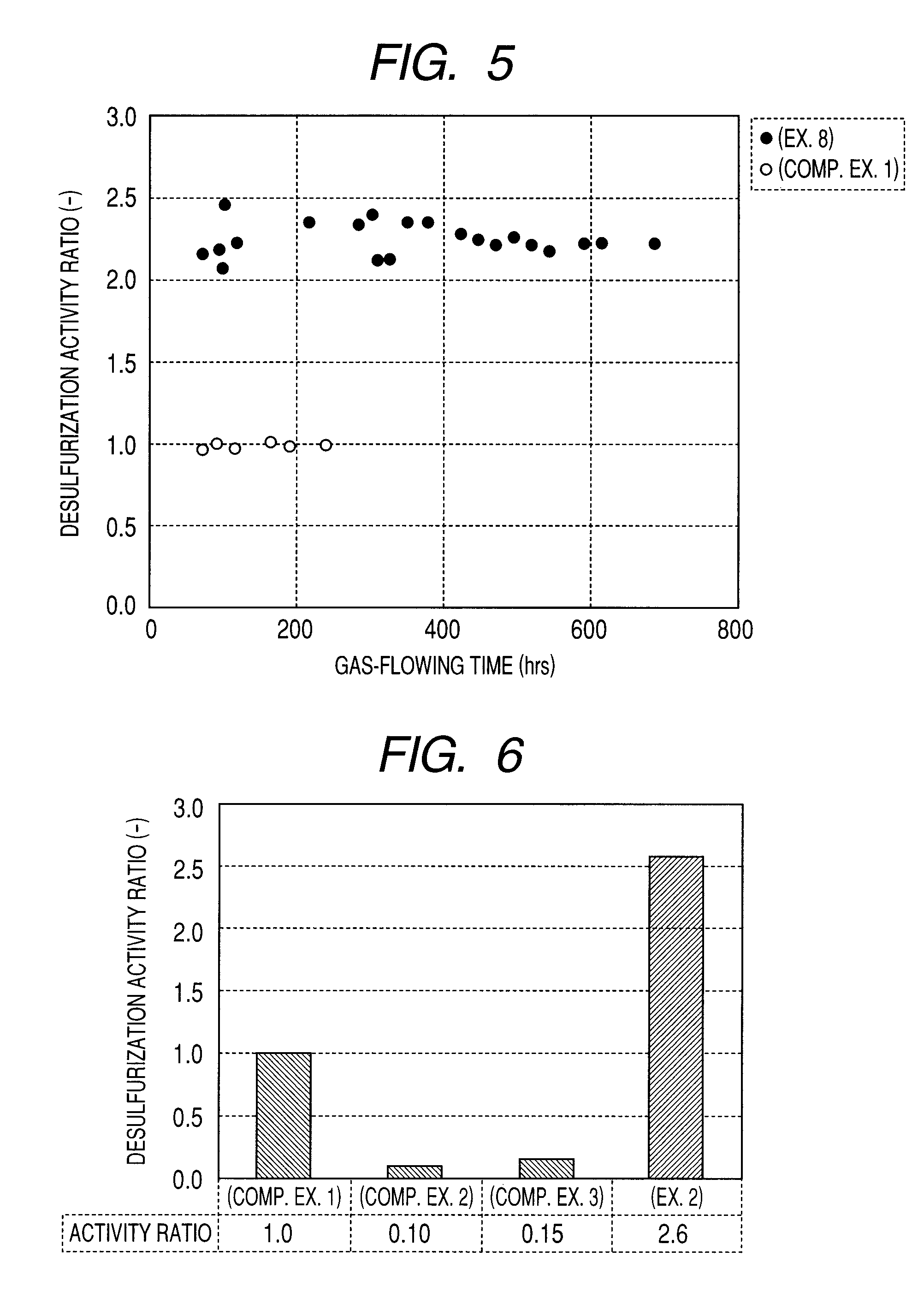
A carbon-based catalyst for flue gas desulfurization is brought into contact with a flue gas containing at least SO2 gas, oxygen and water vapor so that the SO2 gas can react with the oxygen and the water vapor to form sulfuric acid which is to be recovered. On a surface of the carbon-based catalyst, iodine, bromine or a compound thereof is added, ion exchanged or supported and a water-repellent treatment is applied. The carbon-based catalyst can also be used as a mercury adsorbent for flue gas treatment for adsorbing and removing metallic mercury from a flue gas containing metallic mercury, SO2 gas, oxygen and water vapor.
Owner:CHIYODA CORP
Process and apparatus for removal of nitrogen oxides, sulfur oxides and mercury from off gas through oxidization
ActiveUS20130224093A1Lower energy requirementsGas treatmentNitrogen compoundsDecompositionHigh energy
Provided is a process for integrating treatment of off gas through NOx removal, SOx removal and mercury removal, which could avoid high energy consumption resulting from high temperature required for the reaction, and a corresponding apparatus thereof. The process comprises the splitting of the off gas to a sub-stream off gas flow line as a carrier for the oxidizing agent. In the sub-stream line, a heating chamber is set for the decomposition of the oxidizing agent to form free radicals (when hydrogen peroxide is used, the free radicals formed are hydroxy and peroxy hydroxy free radicals) having stronger oxidative capacity than the original oxidants used, these free radicals carried in the sub-stream off gas are then combined with the unheated mainstream off gas, and react with the reducible contents such as NOx, SOx and mercury vapor in the off gas to generate NOx, SOx and mercury ions with higher oxidation state, wherein all the acidic products and mercury ions will be removed in the subsequent gas scrubbing device. The process and apparatus enable substantial reduction of energy required for oxidization and effective removal of NO2 and SOx as well as the reducible heavy metals such as metallic mercury, owing to only a portion of flue gas used as the carrier for the oxidizing and ionizing of the oxidative agents.
Owner:CESTOIL ENVIRO CO LTD
Preparation method of low-mercury compound catalyst for producing vinyl chloride
ActiveCN102500421AExtended service lifeGood dispersionPreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsEvaporationChloride
The invention discloses a preparation method of a low-mercury compound catalyst for producing vinyl chloride, which relates to the catalyst which contains metallic mercury and nickel, and comprises the steps that: firstly a coaly carbon carrier is soaked in aqua regia, and simultaneously flows back in the aqueous solution of triethylamine, is stirred and pre-treated; then surfactant hexadecyltrimethylammonium chloride and additive nickel chloride are added by the mass ratio that hexadecyltrimethylammonium chloride: HgCl2 is 2.85 to 7.65: 4 to 6.5 and NiCl2: HgC12 is 4:4 to 6.5, and impregnated in a vacuum rotary evaporation way, wherein the prepared HgCl2 accounts for 4 percent to 6.5 percent of the total mass of the catalyst and NiCl2 accounts for 4 percent of the total mass of the catalyst, the hexadecyltrimethylammonium chlorid accounts for 2.85 percent to 7.65 percent of the total mass of the catalyst, and the balance is the low-mercury compound catalyst of C, so that the activity, stability and selectivity of the low-mercury compound catalyst are also significantly improved while the mercury content is reduced.
Owner:内蒙古海驰高科新材有限公司
Exhaust gas purifying catalyst
ActiveUS20100183492A1Reduce SO oxidation activityHigh mercury oxidation activityGas treatmentNitrogen compoundsReducing agentOxidation Activity
To overcome the problem of a conventional catalyst and to provide an exhaust gas purifying catalyst that meets the requirement concerning Hg oxidation activity and SO2 oxidation activity; i.e., an exhaust gas purifying catalyst which specifically reduces percent SO2 oxidation, while maintaining percent Hg oxidation at a high level.The invention provides an exhaust gas purifying catalyst which comprises a composition containing oxides of (i) titanium (Ti), (ii) molybdenum (Mo) and / or tungsten (W), (iii) vanadium (V), and (iv) phosphorus (P), wherein the catalyst contains Ti, Mo and / or W, and V in atomic proportions of 85 to 97.5:2 to 10:0.5 to 10, and has an atomic ratio of P / (sum of V and Mo and / or W) of 0.5 to 1.5, and an exhaust gas purifying method comprising exposing an exhaust gas containing a nitrogen oxide (NOX) and metallic mercury (Hg) to the catalyst in the presence of ammonia as a reducing agent, to thereby perform reduction of NOX contained in the exhaust gas and oxidation of metallic mercury (Hg) contained in the exhaust gas.
Owner:MITSUBISHI POWER LTD
Method for rapid detection of mercury content in water
InactiveCN104297174ARapid determinationAvoid relatively large damage, which is easy to cause problems such as blockagePreparing sample for investigationColor/spectral properties measurementsDigestionPrecipitation
The invention discloses a method for rapid detection of mercury content in water. The method includes the following steps: a) preparing a supernatant by an alum precipitation method to prepare a liquid sample to be tested; b) digesting the liquid sample by a sulfuric acid-nitric acid-potassium permanganate digestion method, completely transforming mercury ions into divalent mercury, and reducing excessive oxidant; c) reducing the divalent mercury in the liquid sample to be tested into metallic mercury by using boron hydride; and d) detecting the mercury content in the liquid sample by using a hydride atomic absorption spectrophotometry. The invention provides a determination method with high degree of automation, and can rapidly determine mercury content in a variety of sample sources; and compared with the conventional method, the method is more labor-saving and time-saving in operation, and has high accuracy and high sensitivity.
Owner:SHANGHAI WEIZHENG TEST TECH
Exhaust gas treatment method, exhaust gas treatment system, and catalytic oxidation apparatus
ActiveUS20050112042A1Inhibit deteriorationRestore performanceUsing liquid separation agentCombustible gas catalytic treatmentCombustionCatalytic oxidation
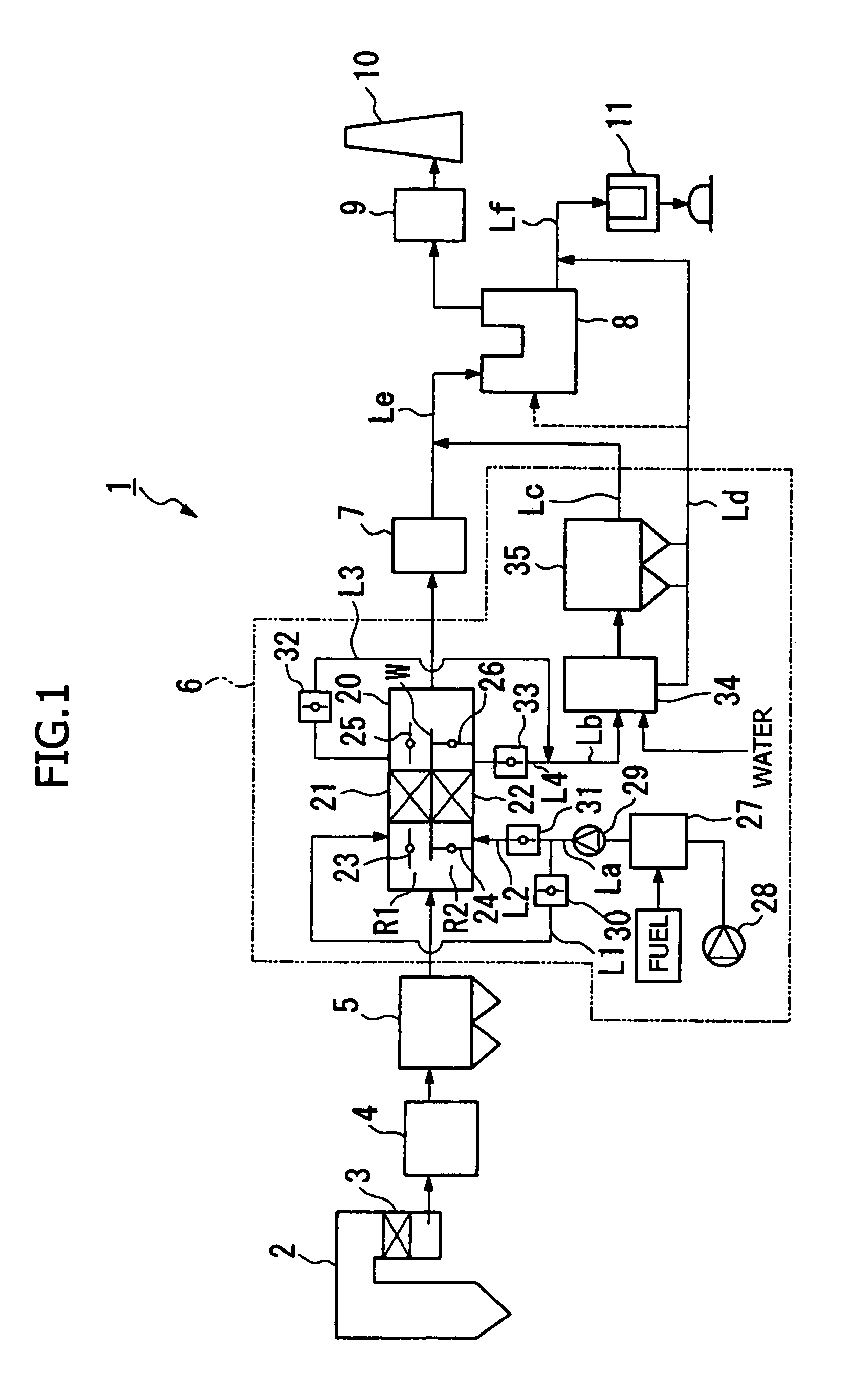
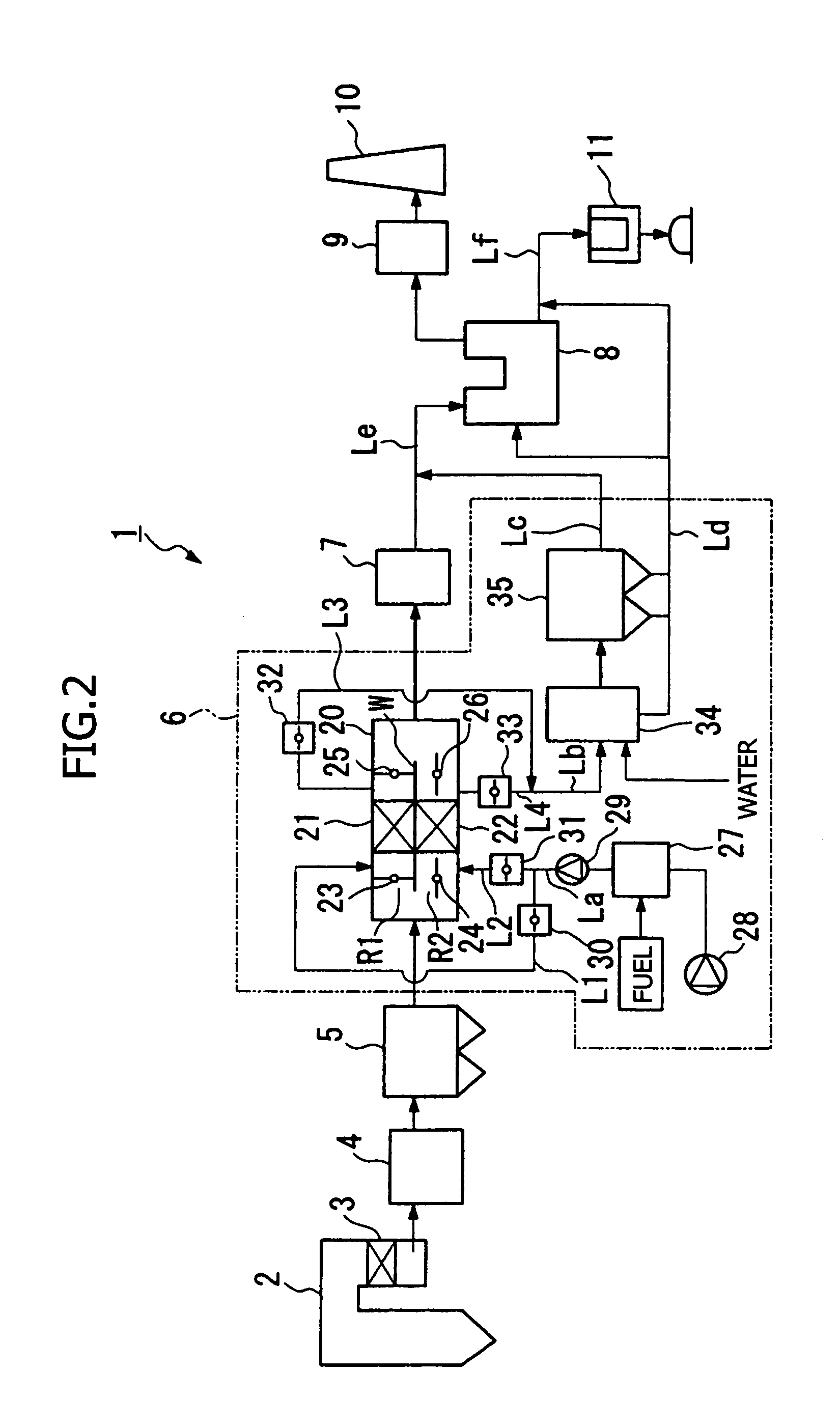
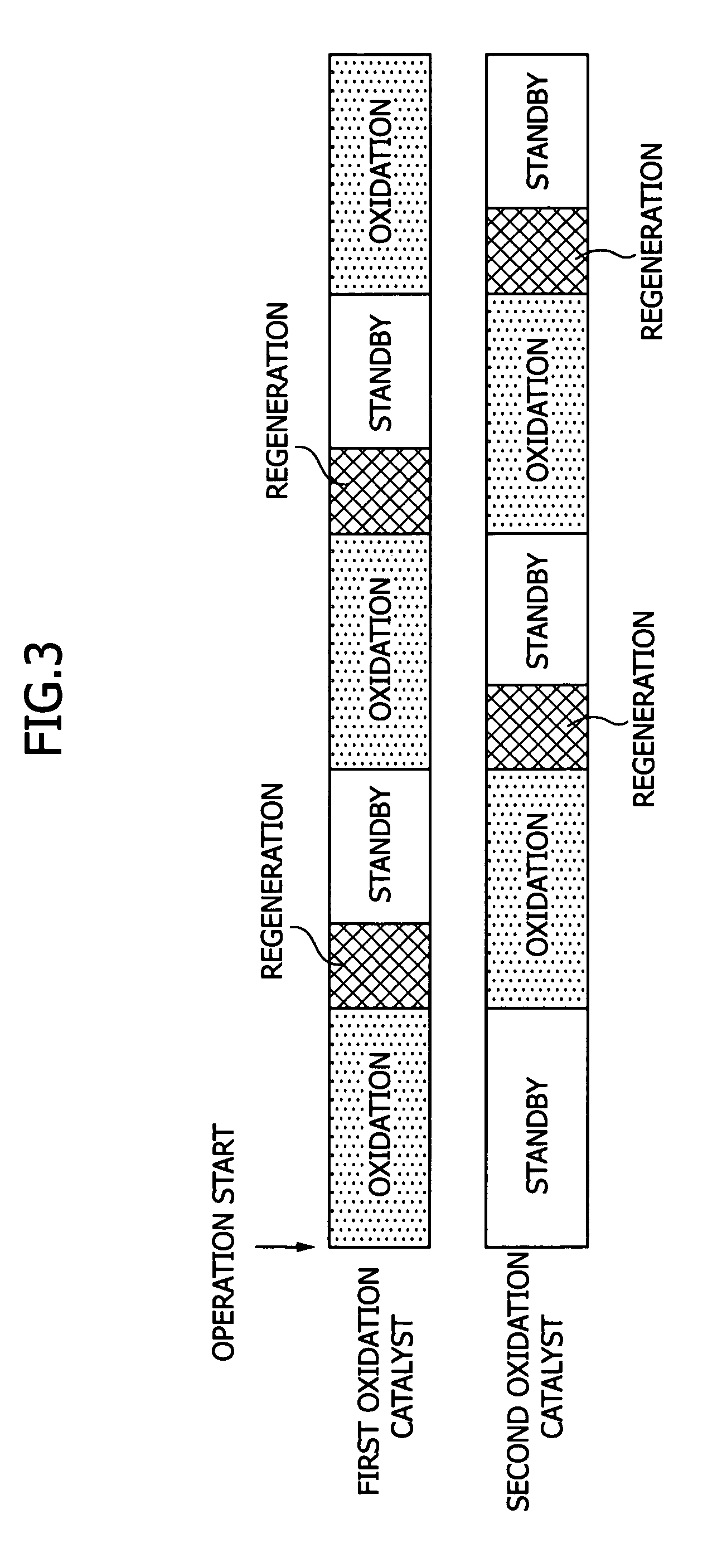
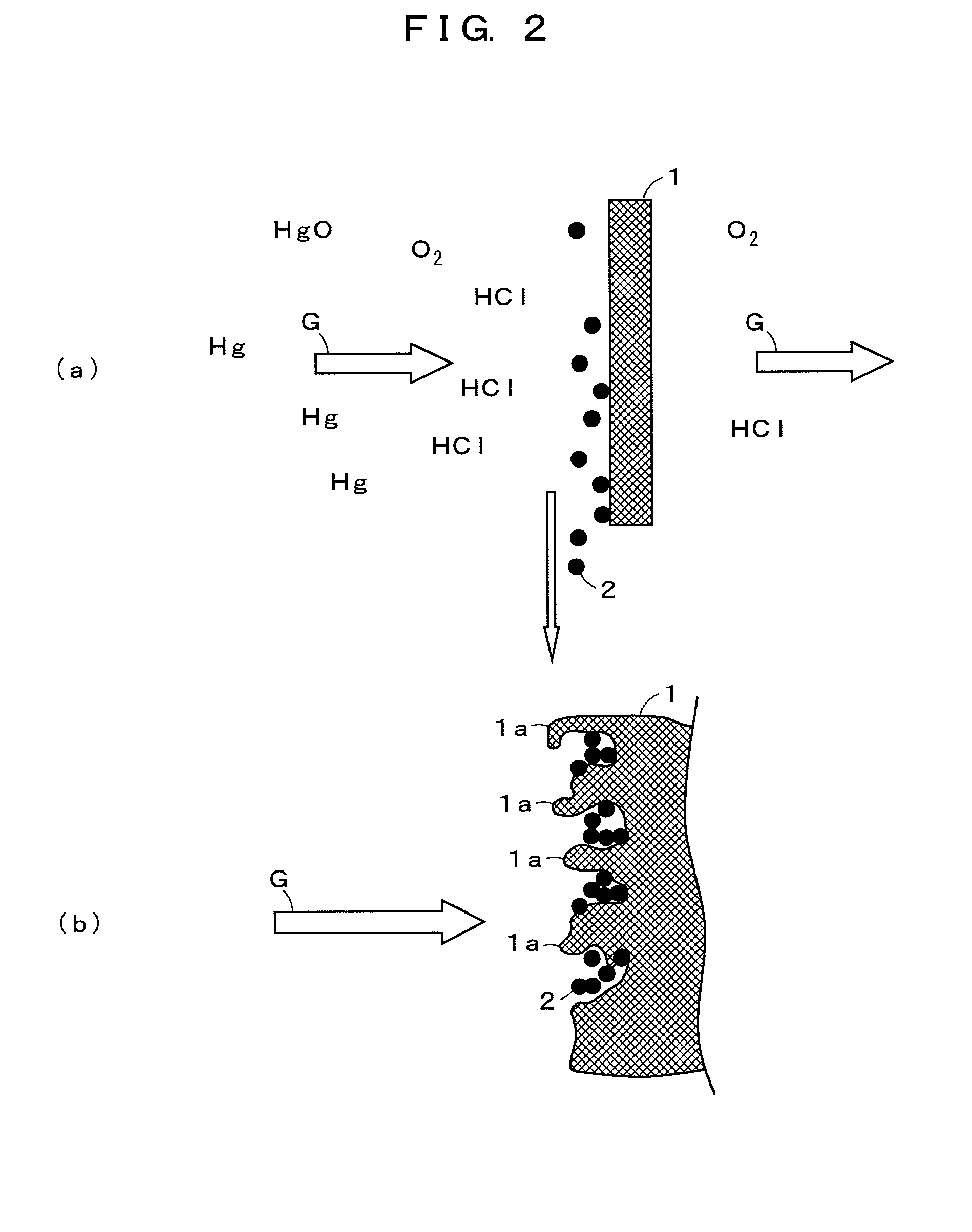
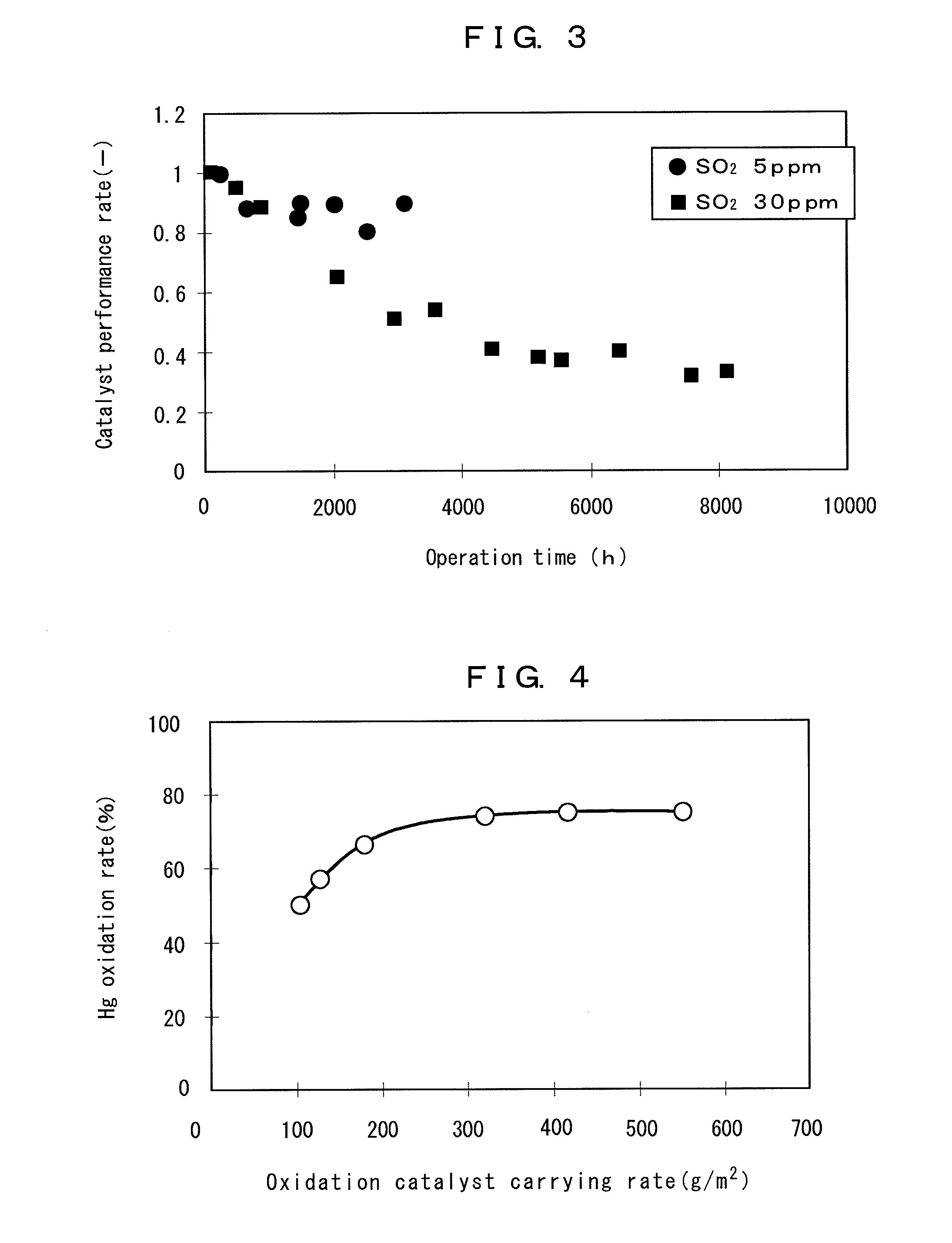
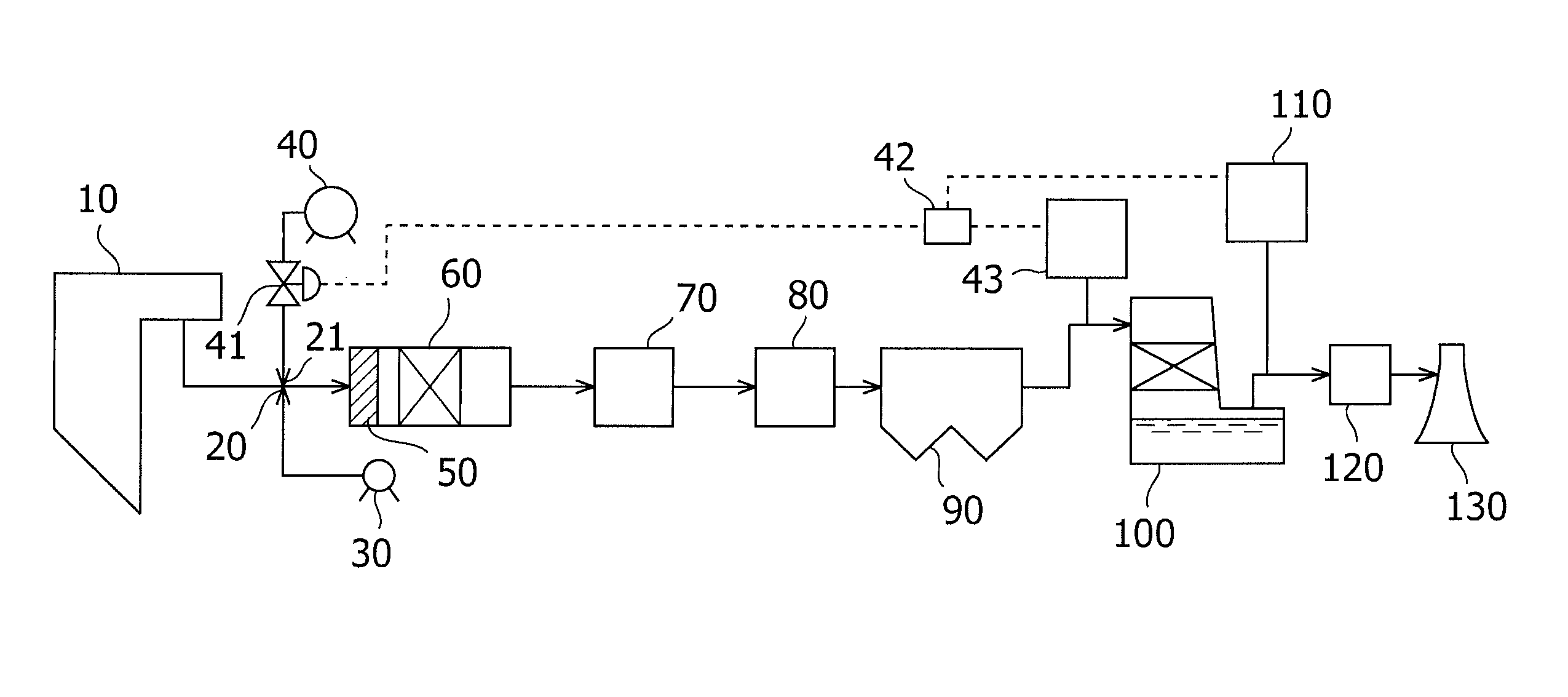
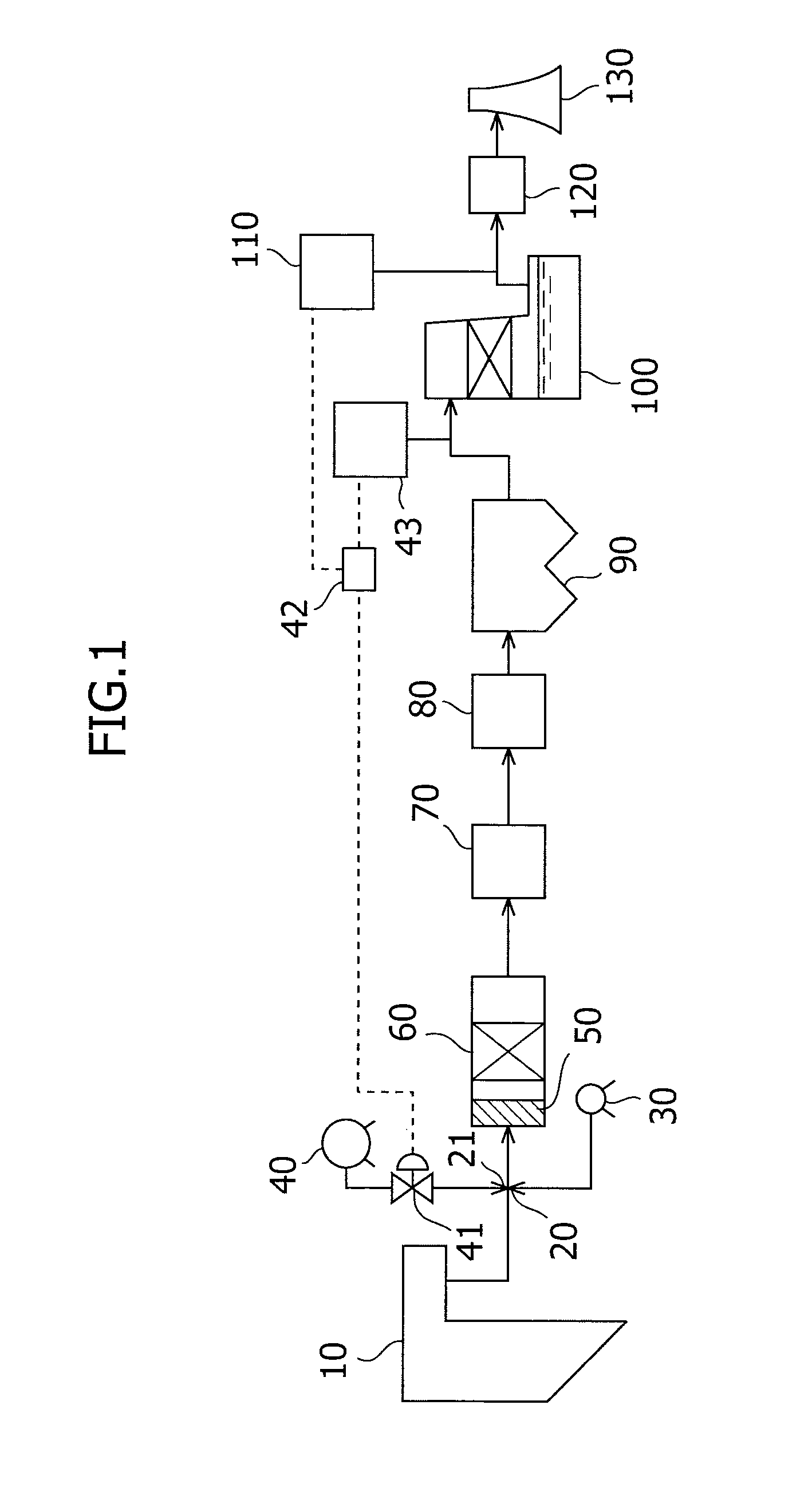
The present invention provides an exhaust gas treatment method including oxidation treatment for converting metallic mercury contained in combustion exhaust gas into mercury chloride; and mercury removal treatment for removing mercury from the combustion exhaust gas by dissolving the mercury chloride in water, wherein a plurality of oxidation catalysts for performing the oxidation treatment are provided, and during the time when the oxidation treatment is performed, at least one of the oxidation catalysts performs catalyst performance restoration treatment without performing oxidation treatment. According to the present invention, the deterioration in mercury removal performance can be restrained even in continuous operation on condition that mercury is oxidized in a low temperature region not higher than 300° C.
Owner:MITSUBISHI HEAVY IND LTD
Preparation method for urine monohydroxyphenyl metabolites detection reagent
InactiveCN102565055APrompt relapsePrompt transferMaterial analysis by observing effect on chemical indicatorMetaboliteBiology
A preparation method for a urine monohydroxyphenyl metabolites detection reagent comprises the steps of preparing solution A, preparing solution B, preparing solution C, uniformly mixing solution A, solution B and solution C at the volume ratio of 1:(1.5-2):(0.5-1.5), adding distilled water into the mixture, and stirring the mixture uniformly, wherein solution A is a mixture prepared through reacting 1 part by weight of metallic mercury with 2-2.2 parts by weight of nitric acid and being diluted by distilled water; solution B is a nickel nitrate solution with the concentration of 0.05g / ml-0.15g / ml; solution C is a mercuric sulfate solution with the concentration of 0.05g / ml-0.15g / ml. The process for preparing testing reagents of monohydroxyphenyl metabolites in urine has the advantages of being low in production cost, and the prepared testing reagents of monohydroxyphenyl metabolites in urine is high in stability and sensitivity, low in false positive rate and false negative rate, and suitable for mass screening and clinical examination.
Owner:宜春中奇金域生物科技股份有限公司
Carbon-based catalyst for flue gas desulfurization and method of producing the same and use thereof for removing mercury in flue gas
ActiveUS20110020205A1Improve desulfurization performanceAmount of catalyst can be reducedGas treatmentOrganic-compounds/hydrides/coordination-complexes catalystsSorbentWater vapor
A carbon-based catalyst for flue gas desulfurization is brought into contact with a flue gas containing at least SO2 gas, oxygen and water vapor so that the SO2 gas can react with the oxygen and the water vapor to form sulfuric acid which is to be recovered. On a surface of the carbon-based catalyst, iodine, bromine or a compound thereof is added, ion exchanged or supported and a water-repellent treatment is applied. The carbon-based catalyst can also be used as a mercury adsorbent for flue gas treatment for adsorbing and removing metallic mercury from a flue gas containing metallic mercury, SO2 gas, oxygen and water vapor.
Owner:CHIYODA CORP
Method for rapid detection of mercury content in ocean sediments
InactiveCN104297173AAvoid relatively large damage, which is easy to cause problems such as blockageHigh degree of automationPreparing sample for investigationColor/spectral properties measurementsDigestionOcean sediment
The invention discloses a method for rapid detection of mercury content in ocean sediments. The method includes the following steps: a) weighing a wet sample of ocean sediments and preparing a liquid sample to be tested; b) digesting the liquid sample by a sulfuric acid-nitric acid-potassium permanganate digestion method, completely transforming mercury ions into divalent mercury, and reducing excessive oxidant; c) reducing the divalent mercury in the liquid sample to be tested into metallic mercury by using boron hydride; and d) detecting the mercury content in the liquid sample by using a hydride atomic absorption spectrophotometry. The invention provides a determination method with high degree of automation, and can rapidly determine mercury content in a variety of sample sources; and compared with the conventional method, the method is more labor-saving and time-saving in operation, and has high accuracy and high sensitivity.
Owner:SHANGHAI WEIZHENG TEST TECH
Method for preparing polythiourea by means of polymerizing multiple components of isocyanide, sulfur and amine and application of polythiourea
ActiveCN108570149AHigh molecular weightAtom economy is highFluorescence/phosphorescenceThioureaFluorescence
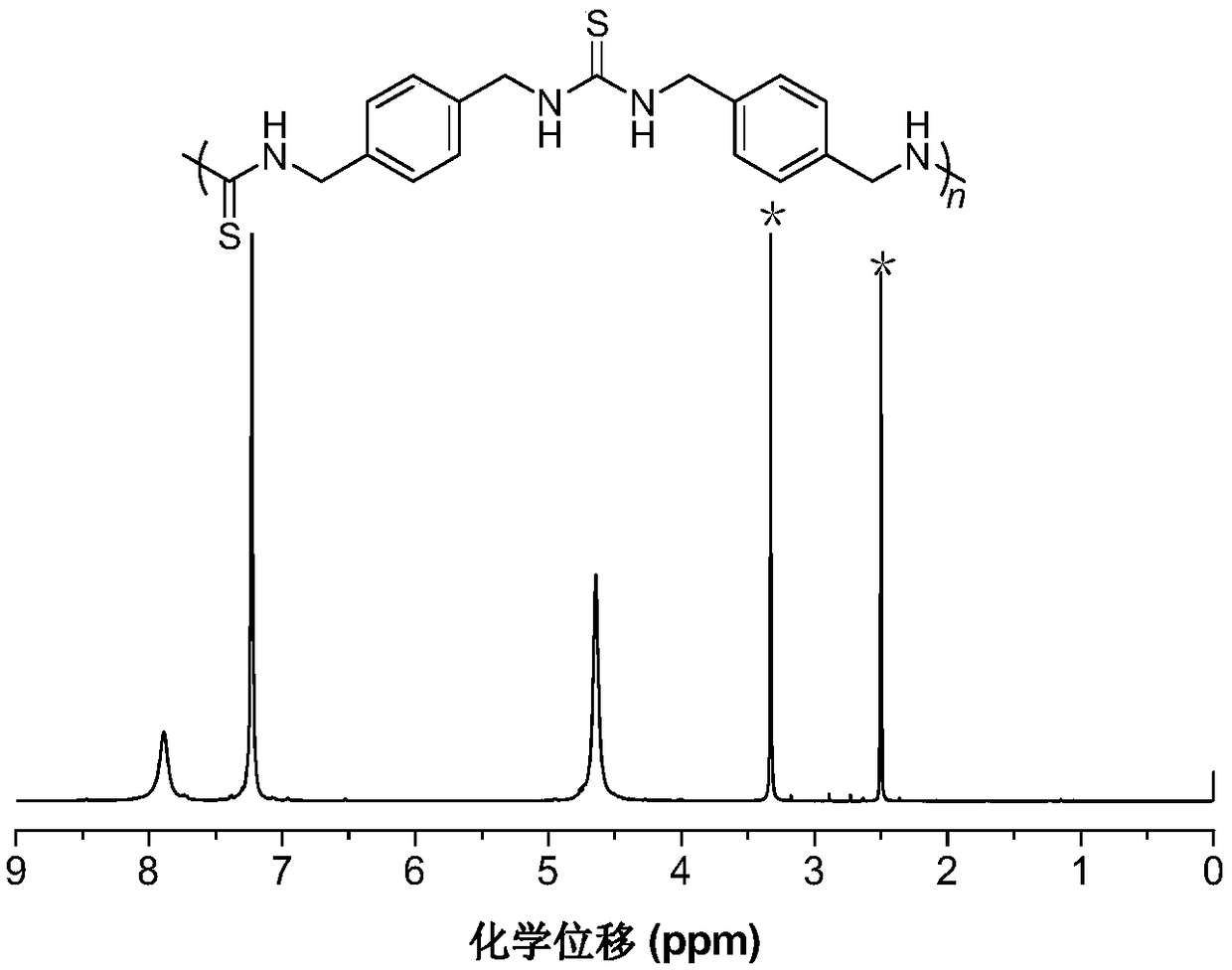
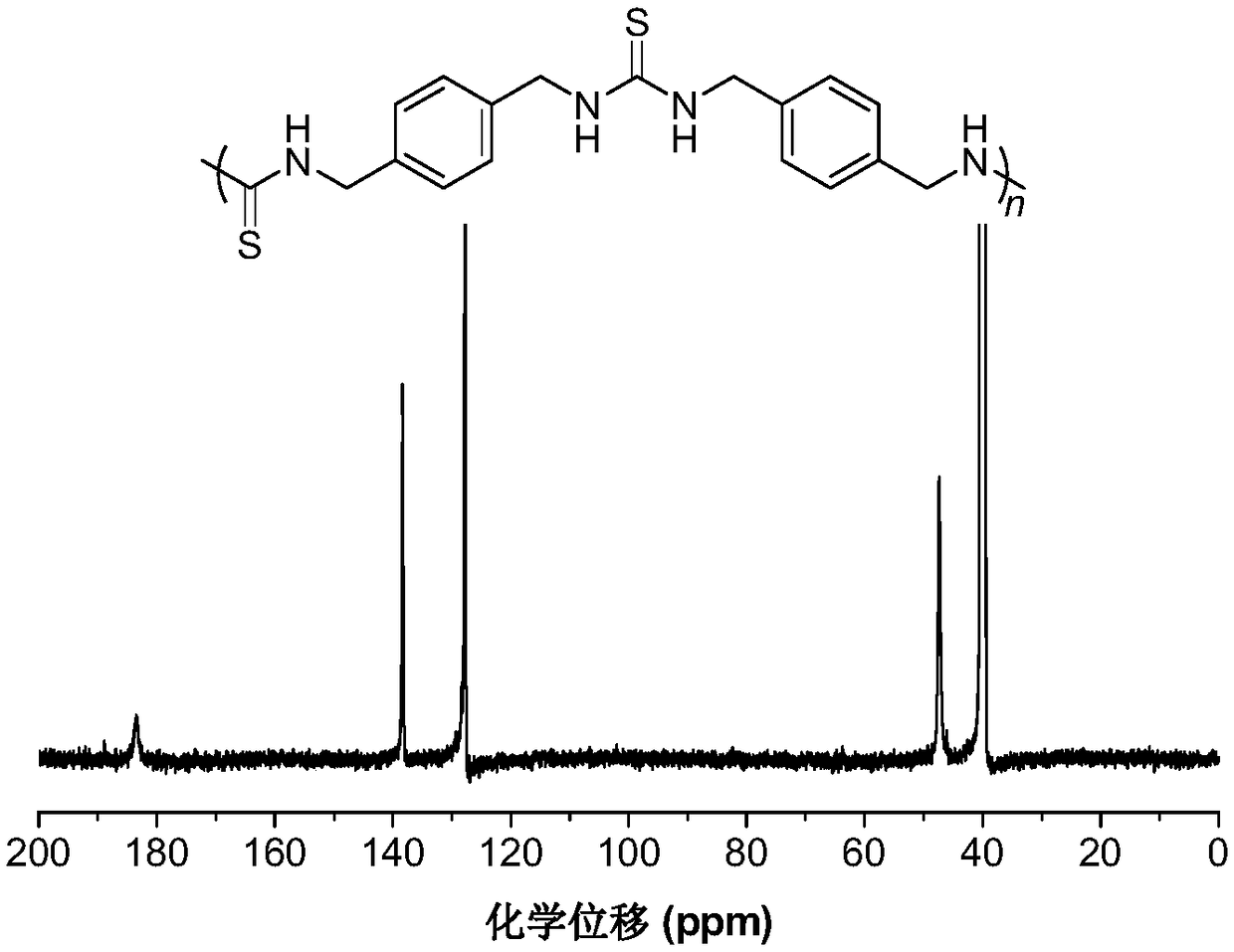
The invention belongs to the technical field of sulfur-containing organic polymer preparation, and discloses a method for preparing polythiourea by means of polymerizing multiple components of isocyanide, sulfur and amine and application of the polythiourea. The method includes carrying out reaction on amine monomers, isocyanide monomers and elemental sulfur in solvents; cooling, precipitating anddrying reaction products to obtain the polythiourea. The amine monomers are diamine compounds, and the isocyanide monomers are binary isocyanide compounds. The method and the application have the advantages that the method can be implemented under room-temperature and air conditions, is simple and is high in polymerization reaction yield and atom economy, and the polythiourea which is a product is easy to separate; the polythiourea prepared by the aid of the method contains abundant N and S heteroatoms, has special photoelectric properties and has a potential unique application value in the field of biological and chemical fluorescence detection, metallic mercury ion detection and mercury ion removal; the polythiourea is high in metallic mercury ion detection sensitivity, good mercury ionremoval effects can be realized by the polythiourea, and the removal efficiency of the polythiourea is higher than 99.99% and can reach drinking water standards.
Owner:SOUTH CHINA UNIV OF TECH
Method and apparatus for themicrobiological removal of mercury from contaminated materials,
InactiveUS20110033913A1Convenient treatmentMercury is reducedBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismElemental mercury
The present invention relates to microorganisms able to reduce mercury ion to metallic mercury; in particular, it refers to systems, apparatuses such as a stirred bioreactor and methods for microbiological mercury removal from contaminated materials, such as, e.g., contaminated environmental matrices, like soil and sediments. The contaminated material is mixed with selected microrganisms capable to enable enzymatic reduction of mercury in ionic form to elemental mercury.
Owner:UNIV DELGI STUDI DI MILANO
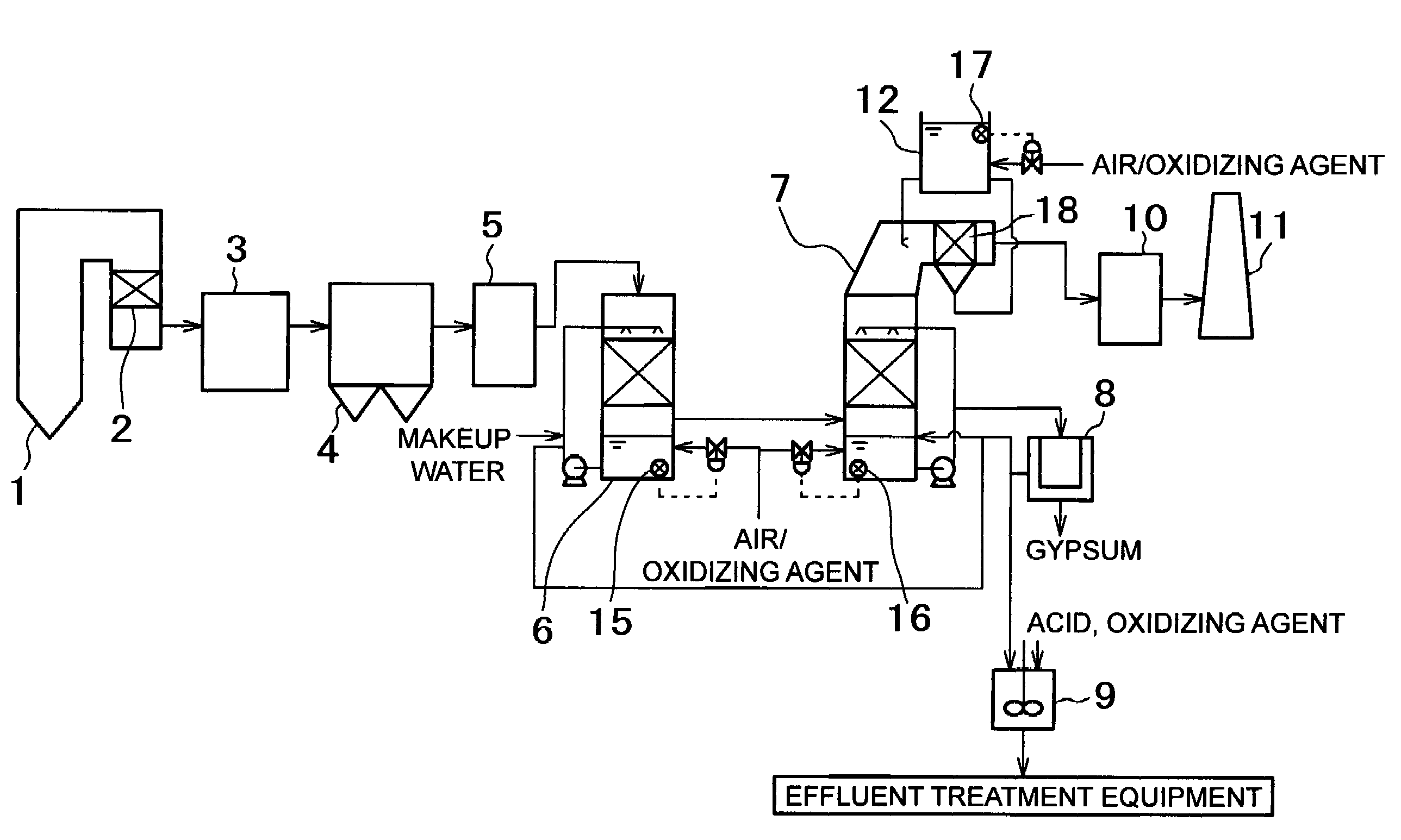
Method for removing mercury in exhaust gas and system therefor
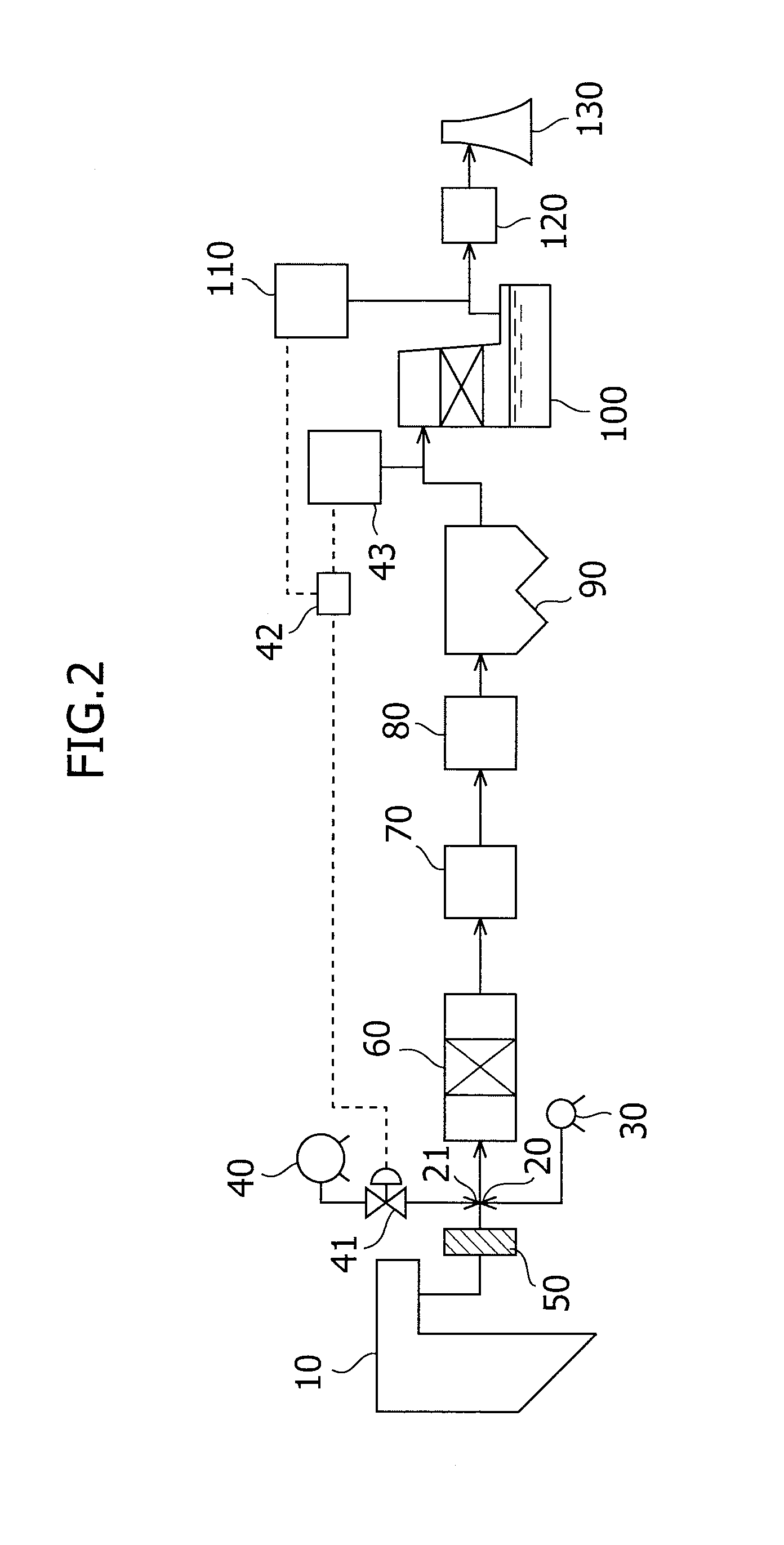
The present invention provides a method for removing mercury in exhaust gas, in which mercury in exhaust gas discharged from combustion equipment is removed, characterized by including a mercury oxidation process in which mercury in the exhaust gas is converted to mercury chloride in the presence of a catalyst; a contact process in which the exhaust gas is brought into contact with an absorbing solution in a scrubber to absorb and remove mercury components from the exhaust gas; and a control process in which blowing of air or addition of an oxidizing agent into the scrubber is accomplished, and the amount of blown air or the added amount of oxidizing agent is regulated to control the oxidation-reduction potential of the absorbing agent, and a system for removing mercury in exhaust gas. According to the mercury removing method in accordance with the present invention, a phenomenon that mercury chloride is reduced into metallic mercury by SO2 etc. and the metallic mercury scatters in the exhaust gas can be prevented, and mercury in the exhaust gas can be decreased effectively.
Owner:MITSUBISHI POWER LTD
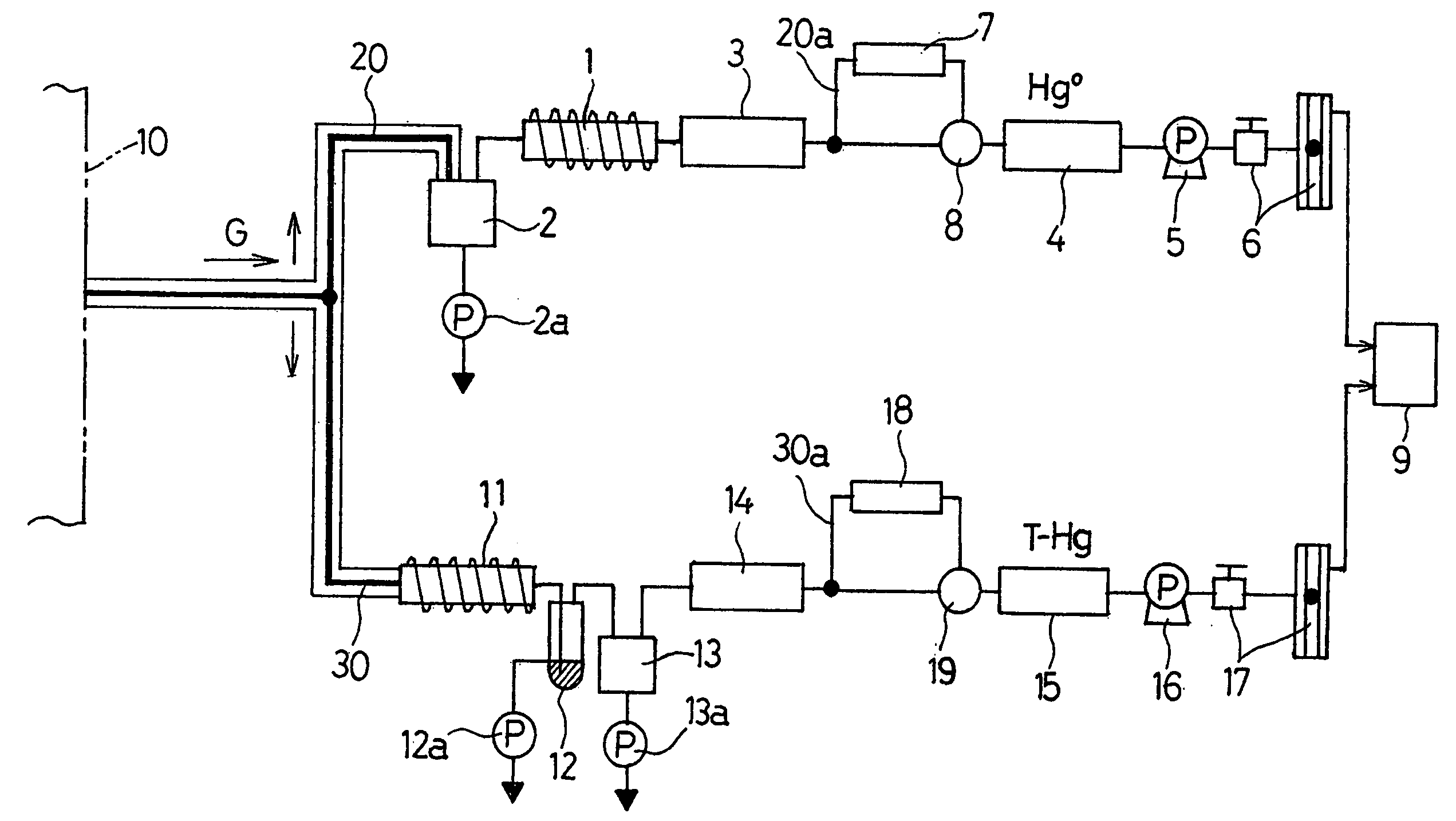
Method of and apparatus for measuring mercury contained in gaseous medium
InactiveUS20040237634A1Simple structureEasy to doChemical analysis using catalysisComponent separationMeasuring instrumentElemental mercury
To provide a method of and an apparatus for continuously measuring elemental mercury and bivalent mercury both contained in a gaseous medium fractionally with a simplified structure, the concentration of a total mercury (Metallic Mercury+Bivalent Mercury) and the concentration of elemental mercury contained in gases are measured continuously and fractionally. In the practice of this mercury measuring method, a first column 1, filled with a first fixed catalyst, and a second column 11, filled with a second fixed catalyst, are fluid connected in parallel relation to each other. The gases G are introduced into those first and second columns 1 and 11. In the first column 1, the first fixed catalyst collects and removes the bivalent mercury, but passes only the elemental mercury in the gases through the first column. In the second column 11, the second fixed catalyst reduces the bivalent mercury into elemental mercury and passes through the second column 11 the elemental mercury in the gases containing the elemental mercury into which the bivalent mercury has been reduced. The concentration of the elemental mercury in the gases, from which the bivalent mercury has been removed after passage thereof through the first column 1 and, also, the concentration of the elemental mercury in the gases into which the bivalent mercury has been reduced after passage thereof through the second column 11 are measured as the concentration of the elemental mercury contained in sampled gases and as the concentration of the total mercury in the sampled gases, respectively by utilization of first and second mercury measuring instruments.
Owner:CENTRAL RESEARCH INSTITUTE OF ELECTRIC POWER INDUSTRY +1
Process and system for removal of contaminants from industrial streams
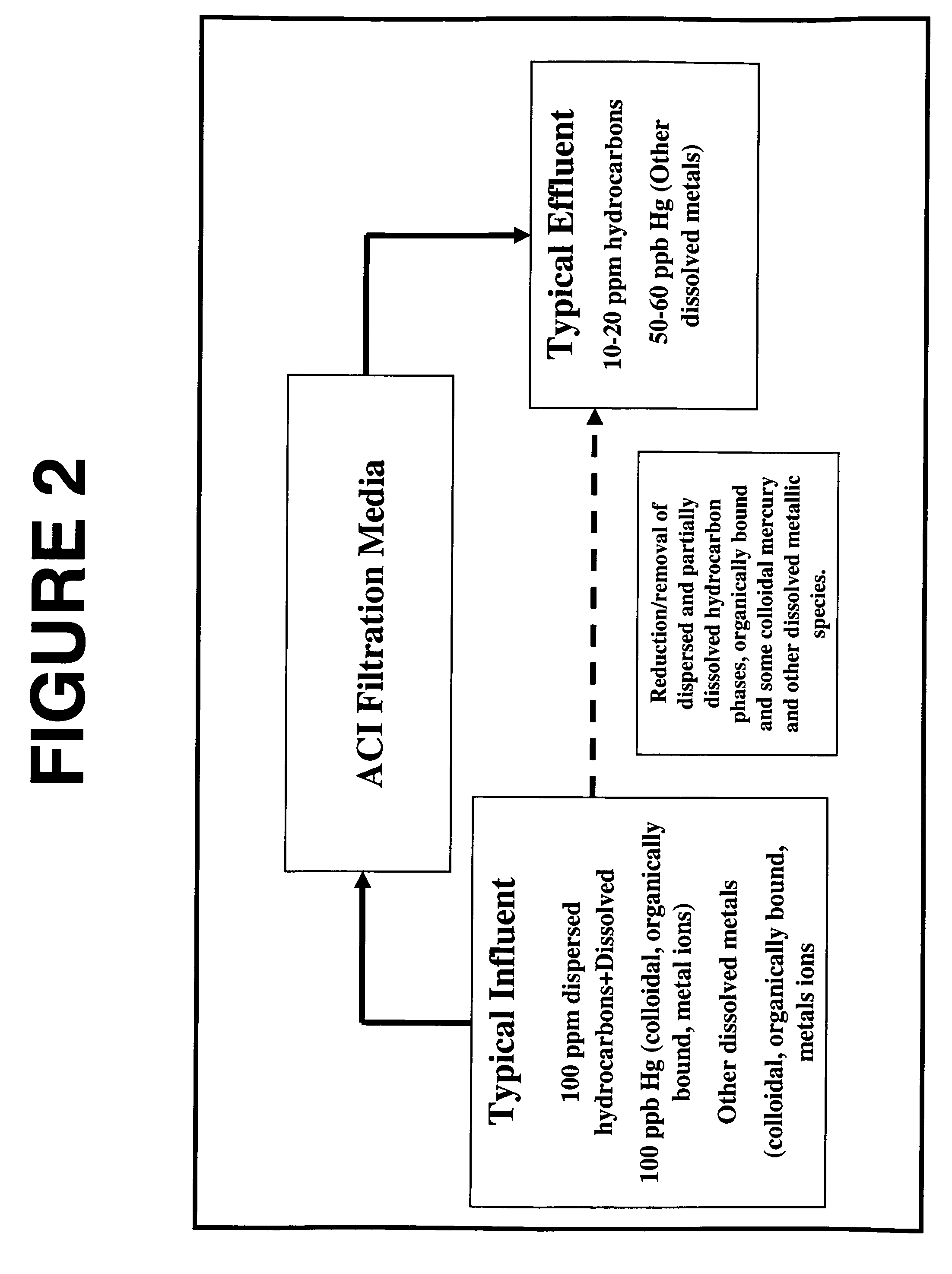
ActiveUS20080210635A1Reduce choiceWater/sewage treatment by irradiationGeneral water supply conservationFiltrationColloid
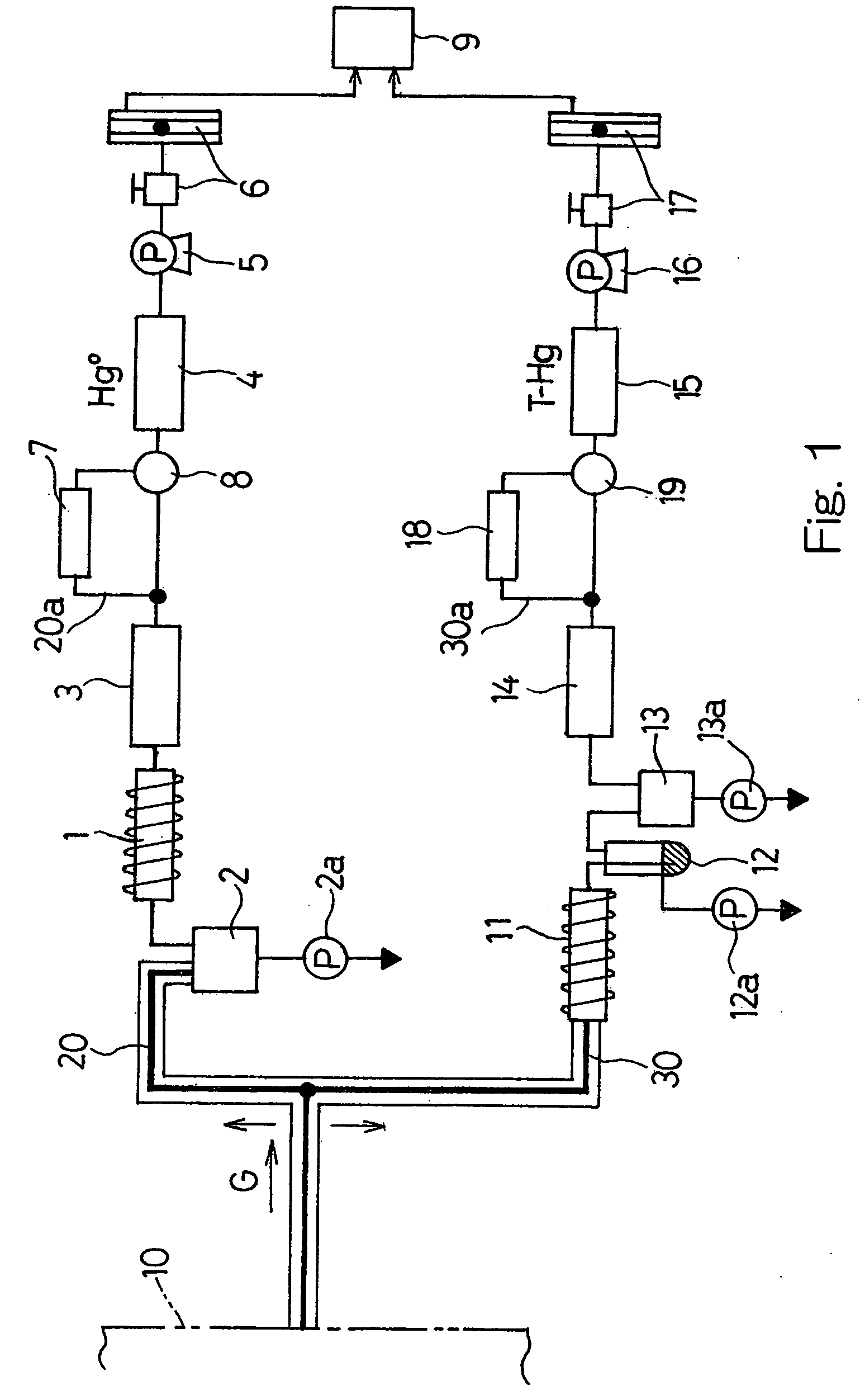
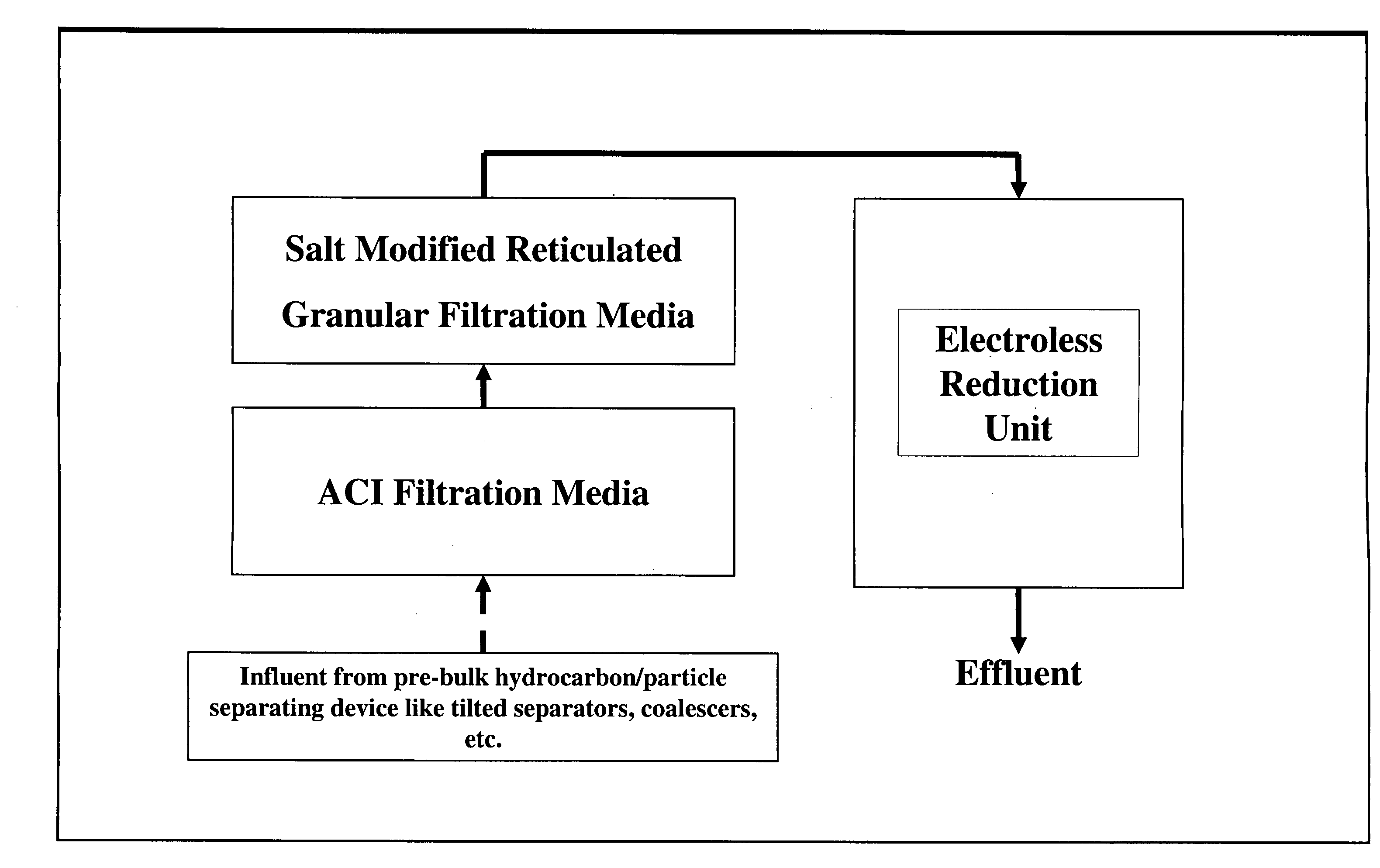
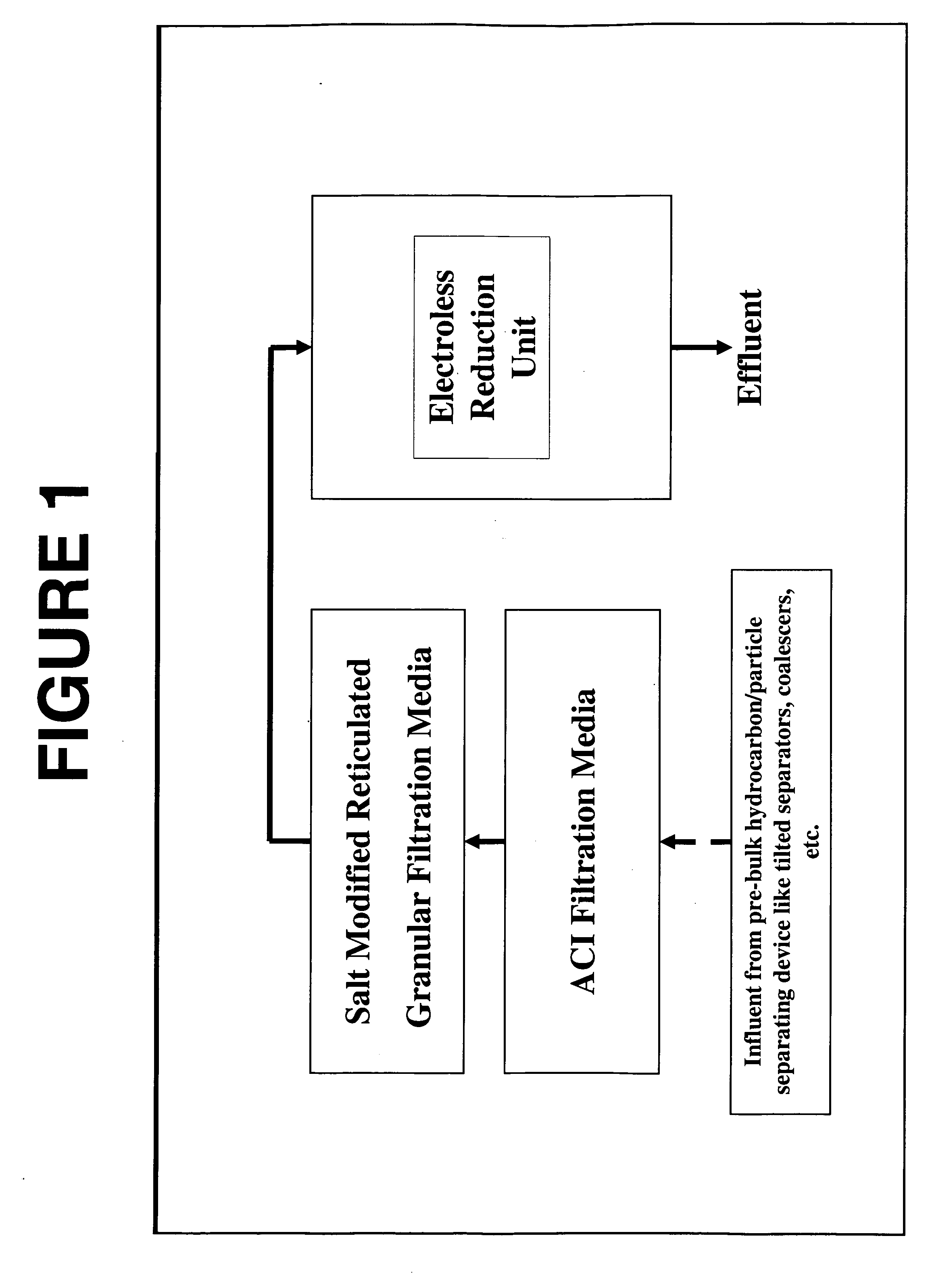
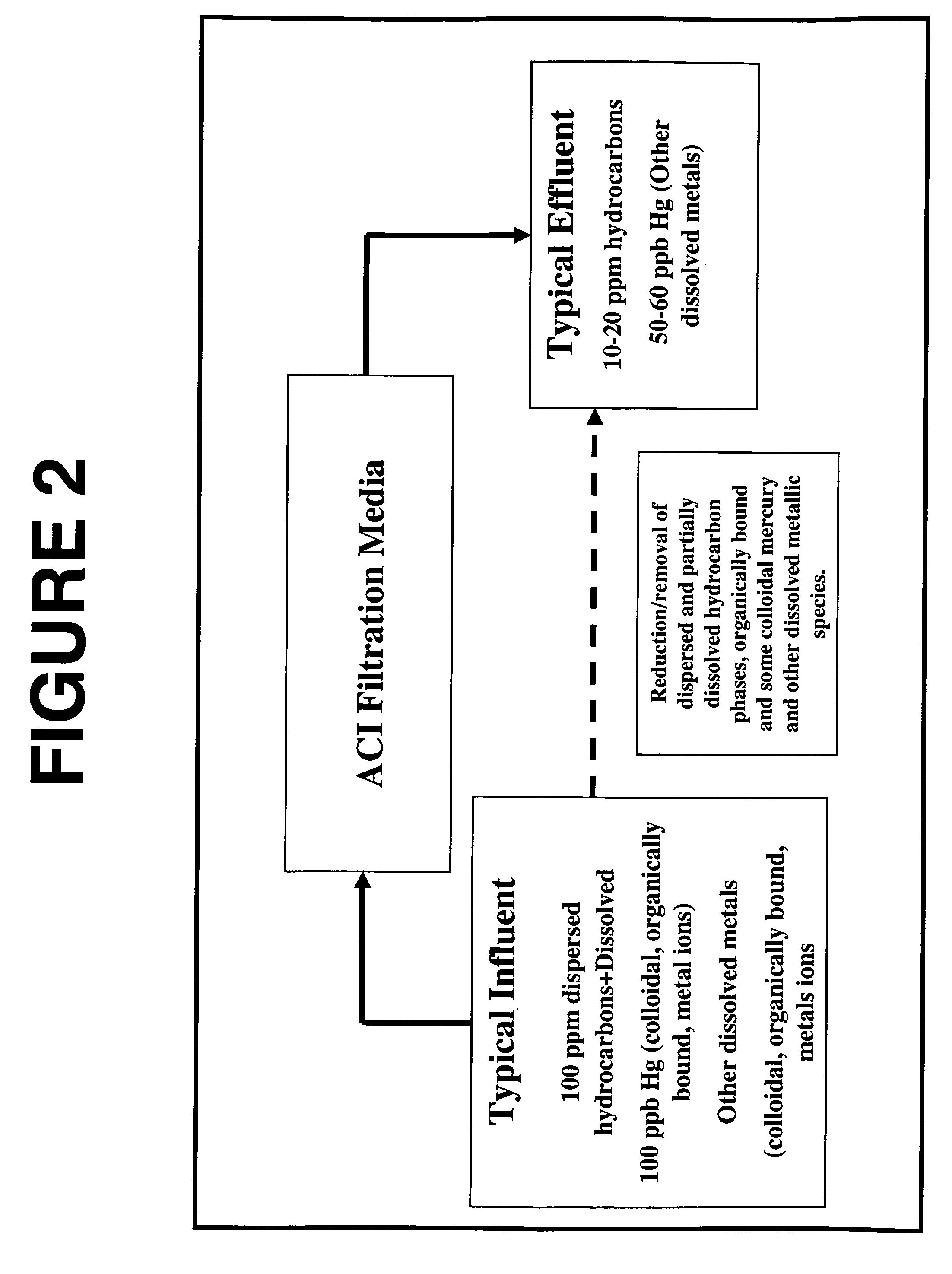
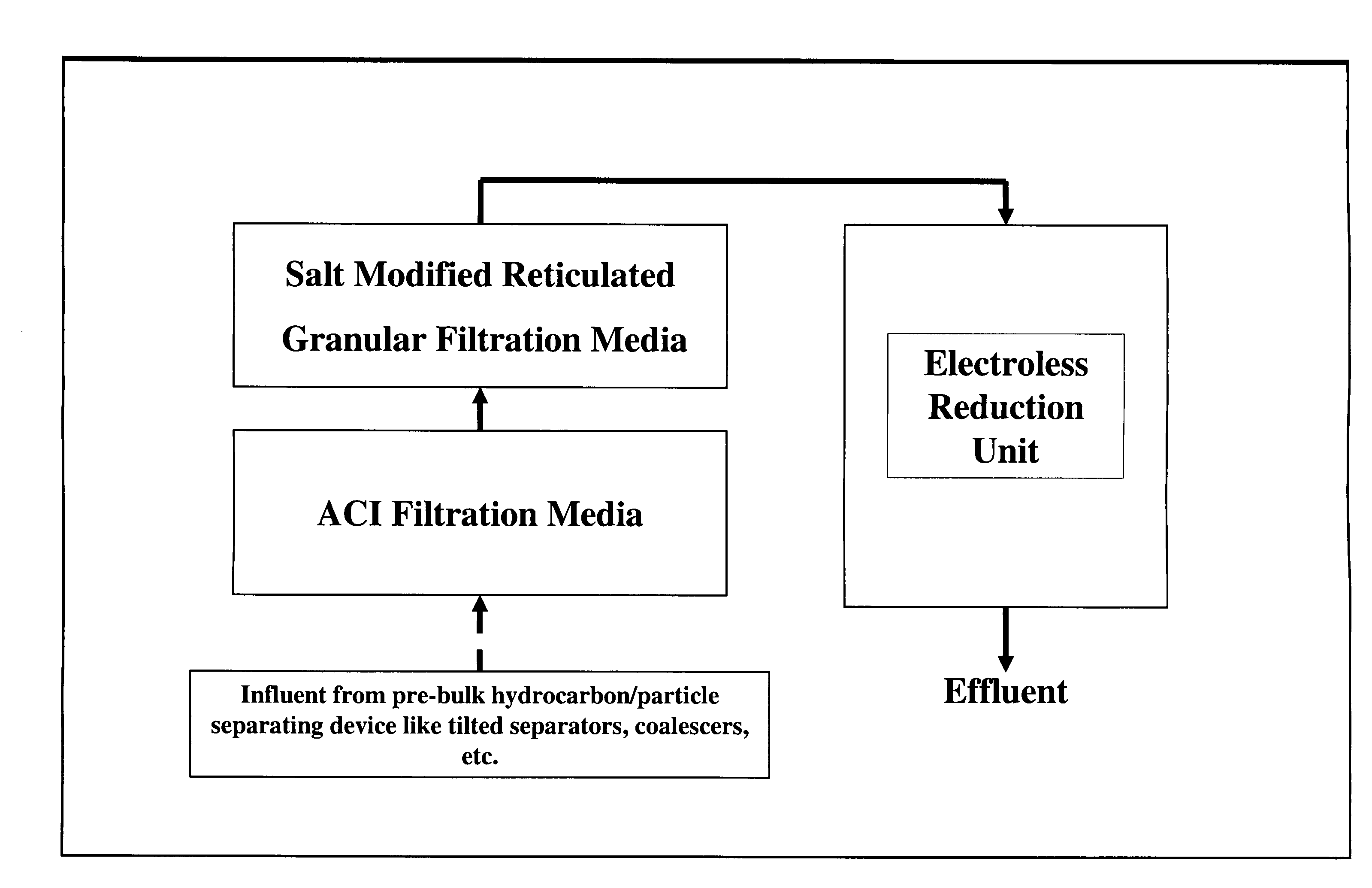
A method and system for removing from an aqueous system which is contaminated therewith: (1) mercury present as colloids, ions and / or organically bound compounds, and (2) hydrocarbons solubilized, dispersed, and / or emulsified in the said system. Pursuant to the invention the aqueous system to be treated (such as “produced water”) is passed successively through three filtration stages. The first filtration stage is provided with absorption media which effects reduction / removal of dispersed organically bound mercury species and of the dispersed and partially dissolved hydrocarbon phases, as well as of some colloidal mercury and other dissolved metallic species. The second filtration stage utilizes a salt modified reticulated granular filtration media for reduction / removal of slightly dissolved hydrocarbon phases, mercury in colloidal and ionic form and other dissolved metals. The third filtration stage is a polishing stage, which serves to further reduce by electroless or voltaic reduction residual elemental mercury and / or residual colloidal and ionic mercury. At this third stage metallic mercury is incorporated into a metallic matrix from which the mercury may preferably be recovered.
Owner:MYCELX TECH CORP
Exhaust gas treatment method, exhaust gas treatment system, and catalytic oxidation apparatus
ActiveUS7235220B2Restore performanceInhibit deteriorationUsing liquid separation agentCombustible gas catalytic treatmentCombustionCatalytic oxidation
The present invention provides an exhaust gas treatment method including oxidation treatment for converting metallic mercury contained in combustion exhaust gas into mercury chloride; and mercury removal treatment for removing mercury from the combustion exhaust gas by dissolving the mercury chloride in water, wherein a plurality of oxidation catalysts for performing the oxidation treatment are provided, and during the time when the oxidation treatment is performed, at least one of the oxidation catalysts performs catalyst performance restoration treatment without performing oxidation treatment. According to the present invention, the deterioration in mercury removal performance can be restrained even in continuous operation on condition that mercury is oxidized in a low temperature region not higher than 300° C.
Owner:MITSUBISHI HEAVY IND LTD
Exhaust gas purifying catalyst
ActiveUS8535628B2Improve performanceImprove oxidation activityCyanogen compoundsNitrogen compoundsPtru catalystNitrogen oxides
To overcome the problem of a conventional catalyst and to provide an exhaust gas purifying catalyst that meets the requirement concerning Hg oxidation activity and SO2 oxidation activity; i.e., an exhaust gas purifying catalyst which specifically reduces percent SO2 oxidation, while maintaining percent Hg oxidation at a high level.The invention provides an exhaust gas purifying catalyst which comprises a composition containing oxides of (i) titanium (Ti), (ii) molybdenum (Mo) and / or tungsten (W), (iii) vanadium (V), and (iv) phosphorus (P), wherein the catalyst contains Ti, Mo and / or W, and V in atomic proportions of 85 to 97.5:2 to 10: 0.5 to 10, and has an atomic ratio of P / (sum of V and Mo and / or W) of 0.5 to 1.5, and an exhaust gas purifying method comprising exposing an exhaust gas containing a nitrogen oxide (NOX) and metallic mercury (Hg) to the catalyst in the presence of ammonia as a reducing agent, to thereby perform reduction of NOX contained in the exhaust gas and oxidation of metallic mercury (Hg) contained in the exhaust gas.
Owner:MITSUBISHI POWER LTD
Apparatus and method for treating discharge gas
ActiveUS20070140939A1Increased cost of treatmentEfficient removal of mercuryCombination devicesGas treatmentTreatment costsOxide sulfur
The invention provides a discharge gas treatment apparatus and method which prevent increase in treatment cost and which attain efficient removal of mercury contained in a discharge gas. The discharge gas treatment apparatus, for treating a discharge gas containing nitrogen oxide, sulfur oxide, metallic mercury, and hydrogen chloride, includes a cooling apparatus for controlling the temperature of the discharge gas to a predetermined temperature in accordance with the hydrogen chloride concentration of the discharge gas; an NOx removal catalyst unit for reducing nitrogen oxide contained in the discharge gas for removal thereof and for causing reaction between mercury and hydrogen chloride contained in the discharge gas to thereby form mercury chloride, the discharge gas having been controlled to the predetermined temperature and having been introduced to the apparatus, with ammonia being added thereto; and a wet-format desulfurization apparatus, disposed on the downstream side with respect to the NOx removal catalyst unit, for removing sulfur oxide and mercury chloride contained in the discharge gas through dissolving sulfur oxide and mercury chloride in a liquid absorbent. The apparatus has a simple structure and prevents an increase in treatment cost.
Owner:MITSUBISHI HEAVY IND LTD
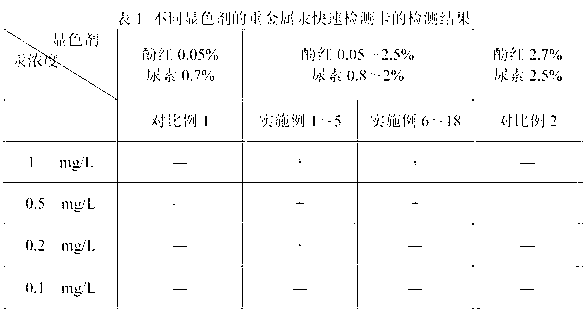
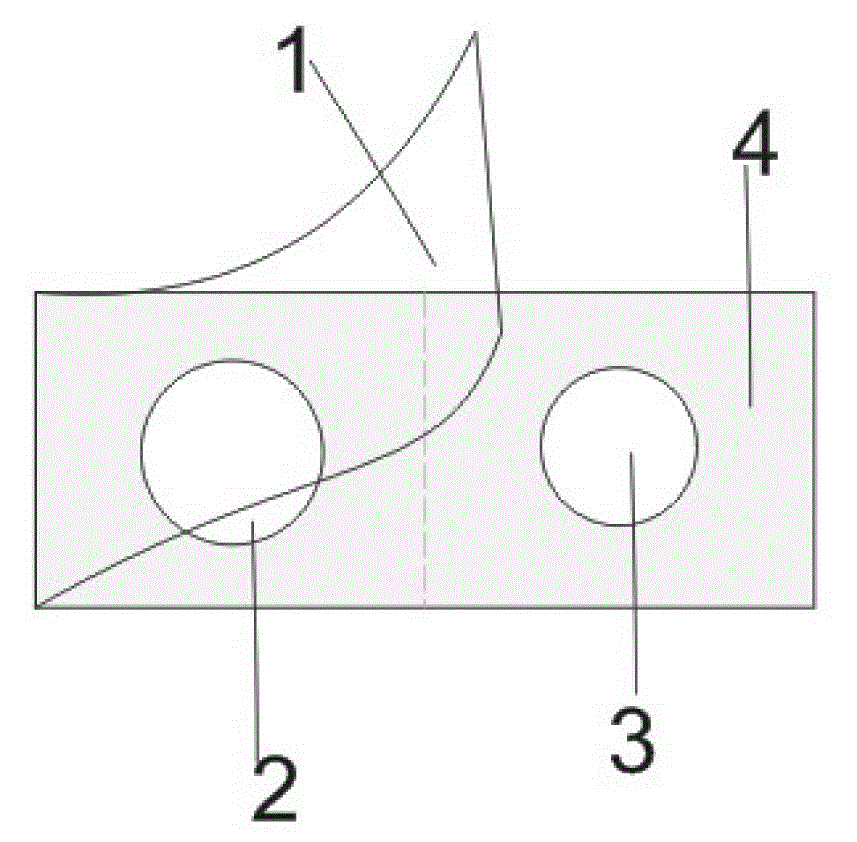
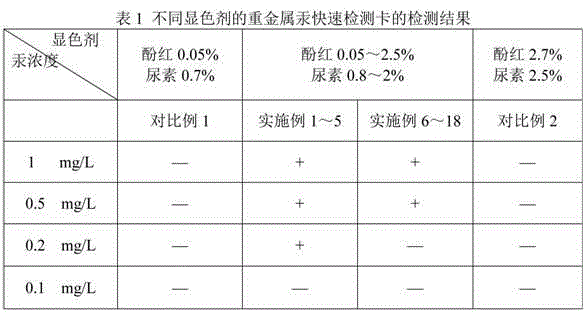
Rapid testing card for heavy metal mercury and testing method thereof
ActiveCN102776266ALow costSimple and fast operationMicrobiological testing/measurementPhysical chemistryEngineering
The invention discloses a rapid testing card for heavy metal mercury and a testing method thereof. The structure of the testing card includes a base board, absorption wafers and a cover film successively from bottom to top. At least one wafer for absorbing color-developing agents and at least one wafer for absorbing urease are respectively fixed on the base board, and the wafers are not overlapped mutually. The use amount ratio of the urease and the color-developing agents absorbed by the wafer per unit area is 3U: (1.6-3.2mL), and the color-developing agents contain components of, by mass, 0.05% to 2.5% of phenol red and 0.8% to 2% of urea. The testing card can test the content of mercury larger than or equal to 0.2 mg / L rapidly, accurately and conveniently; the test card is low in cost, simple and convenient to operate, short in testing time, in no need of professionals, sensitive in reaction and easy to observe and interpret; and the testing card is non-toxic and harmless and cannot cause environment pollution.
Owner:广东中检达元检测技术有限公司
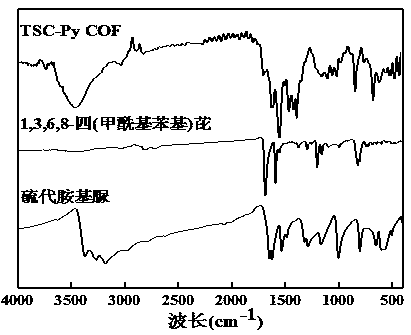
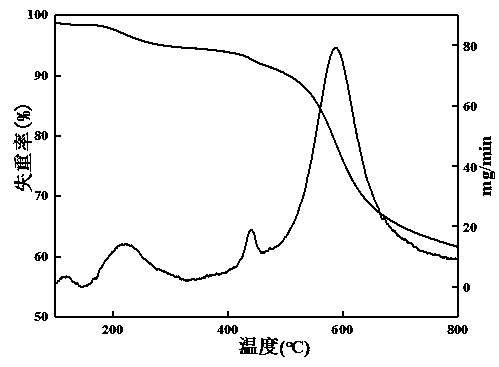
Synthesis and application of porous covalent organic framework material with imine structure
InactiveCN109054039AImprove thermal stabilityGood chemical stabilityFluorescence/phosphorescenceLuminescent compositionsAcetic acidFluorescence
The invention provides a synthesis method for a covalent organic framework material with an imine structure. The method comprises the following steps: using thiosemicarbazide and 1,3,6,8-tetrabenzoylpyrene as raw materials, using o-dichlorobenzene and N,N-dimethylacetamide as solvents, and using acetic acid as a catalyst, synthesizing to obtain TSC-Py COF through a solvothermal method. In DMF solution of the TSC-Py COF material, metal ion solutions of Ag<+>, Co<2+>, Fe<3+>, Cr<3+>, Pb<2+>, Zn<2+>, Na<+>, Mg<2+>, Mn<2+>, Ca<2+>, La<3+>, Ni<2+>, Al<3+>, Cu<2+>, Ce<3+>, Ba<2+>, K<+>, Cd<2+>, Pr<3+>, Fe<2+>, and Hg<2+> are respectively added. Only if mercury ions are added, the TSC-Py COF solution has the good fluorescence enhancement effect, so the covalent organic framework material can beused for fluorescence-detecting the metallic mercury ions.
Owner:NORTHWEST NORMAL UNIVERSITY
Microbiological method for detecting metallic mercury in water body
InactiveCN104845996ALow fluorescent expressionSuperiorBacteriaMicrobiological testing/measurementEscherichia coliFluorescence
The invention provides a construction method for an engineering strain of Escherichia coli used for detecting the heavy metal mercury and establishment of a method for detecting mercury in a water body by using the engineering strain of Escherichia coli. The genetic engineering strain is merR:: PmerT:: cfp-linker-cfp / E.coli delta zntA, named as E.coli WMC-010 (CTMCC No. 9749), wherein mer operon originated from Serratia marcescens is knocked into the zntA position of the genome of a wild E. coli MC4100 strain to start expression of cyan fluorescent protein (CFP). The engineering strain provided by the invention can overcome the defects of a high fluorescence background value, poor independent experiment repeatability and difficulty in accurate quantification of the heavy metal mercury in a water body of a reporter strain containing reporter plasmid and can reflect bio-availability of mercury in a water body, so bases are provided for objective evaluation of the biotoxicity of the heavy metal mercury in a water body.
Owner:WENZHOU MEDICAL UNIV
Apparatus for removing of trace of toxic substance from exhaust gas and method of operating the same
ActiveUS20100000410A1Reduce the temperaturePromote absorptionCombination devicesGas treatmentAir preheaterCombustion
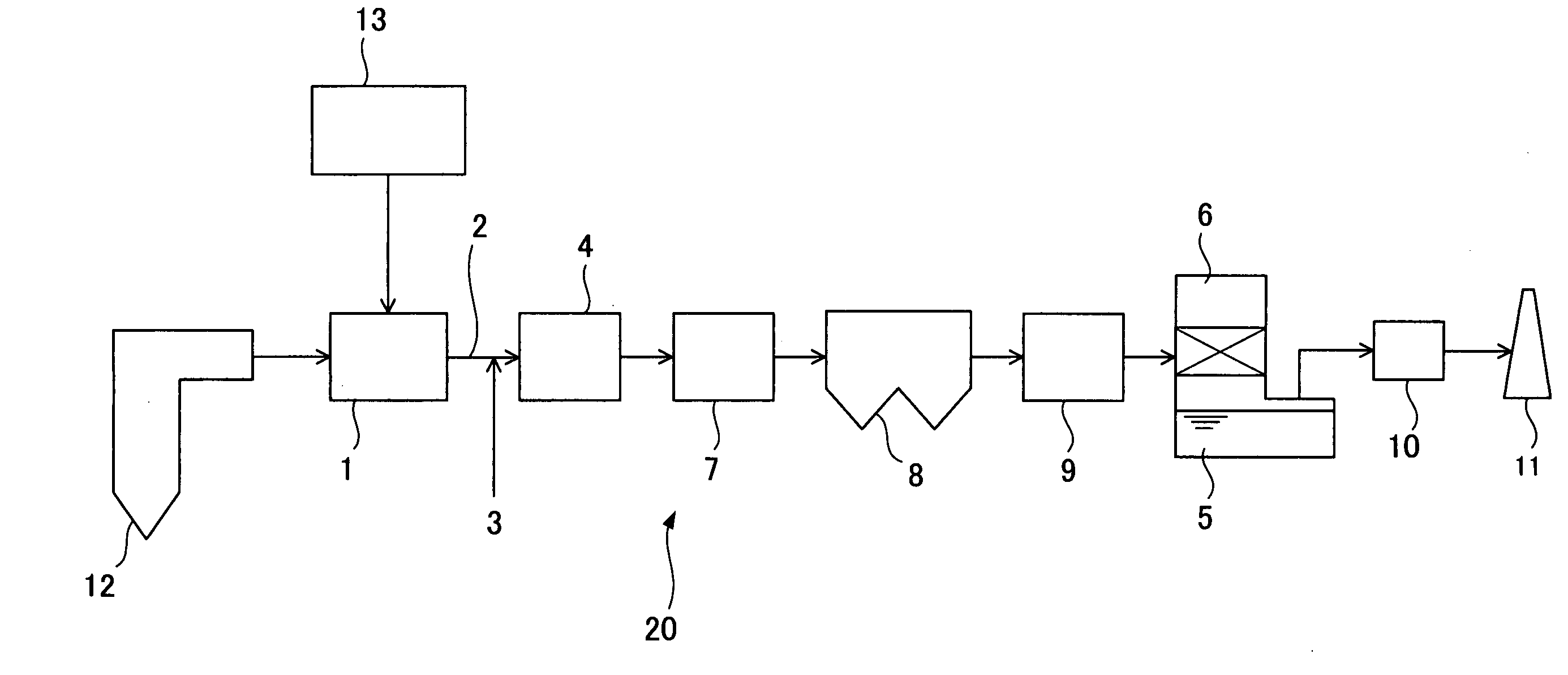
An apparatus for removing of traces of toxic substances from exhaust gas, comprising, disposed in sequence from the upstream side in a flow channel of exhaust gas emitted from combustion equipment, a denitration unit including a denitration catalyst layer capable of removing nitrogen oxides from the exhaust gas and capable of oxidizing metallic mercury; an air preheater adapted for heat exchange between air for combustion in the combustion equipment and the exhaust gas; a dust removal unit having a bag filter containing a catalyst for metallic mercury oxidation; and a desulfurization unit for removing sulfur oxide from the exhaust gas. The bag filter may be disposed in advance of the desulfurization unit. Thus, there can be provided an apparatus for removing of traces of toxic substances from exhaust gas that is stable over a prolonged period of time and is highly reliable; and provided a method of operating the same.
Owner:MITSUBISHI POWER LTD
Exhaust gas purifying catalyst
ActiveUS20130129590A1Reduce SO oxidation activityHigh mercury oxidation activityCyanogen compoundsNitrogen compoundsReducing agentOxidation Activity
To overcome the problem of a conventional catalyst and to provide an exhaust gas purifying catalyst that meets the requirement concerning Hg oxidation activity and SO2 oxidation activity; i.e., an exhaust gas purifying catalyst which specifically reduces percent SO2 oxidation, while maintaining percent Hg oxidation at a high level.The invention provides an exhaust gas purifying catalyst which comprises a composition containing oxides of (i) titanium (Ti), (ii) molybdenum (Mo) and / or tungsten (W), (iii) vanadium (V), and (iv) phosphorus (P), wherein the catalyst contains Ti, Mo and / or W, and V in atomic proportions of 85 to 97.5:2 to 10: 0.5 to 10, and has an atomic ratio of P / (sum of V and Mo and / or W) of 0.5 to 1.5, and an exhaust gas purifying method comprising exposing an exhaust gas containing a nitrogen oxide (NOX) and metallic mercury (Hg) to the catalyst in the presence of ammonia as a reducing agent, to thereby perform reduction of NOX contained in the exhaust gas and oxidation of metallic mercury (Hg) contained in the exhaust gas.
Owner:MITSUBISHI POWER LTD
Method and apparatus for treating exhaust gas
ActiveUS8420034B2Reduce the amount requiredCurb energy consumptionGas treatmentMagnesium halidesAmmoniaMetal
Owner:MITSUBISHI HEAVY IND LTD
Fe3O4-rGO (reduced graphene oxide)-Ag composite material, method for preparing same and application of Fe3O4-rGO-Ag composite material
InactiveCN107029667AImprove adsorption capacityAchieving Removal EfficiencyGas treatmentOther chemical processesActive componentEconomic benefits
The invention discloses an Fe3O4-rGO (reduced graphene oxide)-Ag composite material. Active components Fe3O4 and Ag are loaded on graphene oxide (GO) which is a carrier to obtain the Fe3O4-rGO-Ag composite material. The invention further discloses a method for preparing the Fe3O4-rGO-Ag composite material and application thereof. The Fe3O4-rGO-Ag composite material, the method and the application have the advantages that characteristics of large specific surface areas and multiple functional groups of reduced graphene oxide are utilized, silver and iron which are good in adsorption performance can be loaded, the silver is precious metal, the iron is a magnetic material, and accordingly the zero-valent mercury removal efficiency can reach 90% at least; magnetic composite adsorption materials can be separated and recycled by magnetic separation means, can be regenerated at the high temperatures and then can be reused, metal mercury with extremely high purity can be recycled, accordingly, the operation cost can be reduced to a great extent, and the Fe3O4-rGO-Ag composite material and the method can have direct economic benefits.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Simultaneous process of electroreducing ytterbium and oxidizing cerium
InactiveCN1986895APrevent volatilizationPrevent dispersalPhotography auxillary processesProcess efficiency improvementCeriumIon-exchange membranes
The present invention is one electrolytic process of reducing Yb, enriching Tu and Lu, and oxidizing Ce simultaneously. The electrolytic process reduces Yb into bivalent Yb ion in the cathode chamber so as to separate out Yb from the enriched Yb, Tu and Lu; oxidizes Ce into four-valent ion so as to separate out Ce. The electrolytic equipment has cathode of metal mercury or amalgam, anode of inert metal, anion exchange membrane to separate the anode chamber from the cathode chamber, cathode solution of sulfuric acid solution of pH 0.1-4.0 with enriched Yb, Tu and Lu, and anode solution of sulfuric acid solution of acidity 0.01-3.0 mol / L with enriched Ce. The present invention has low production cost, best utilization of electrolytic current and other advantages.
Owner:北京方正稀土科技研究所有限公司 +2
Process for removal of contaminants from industrial streams
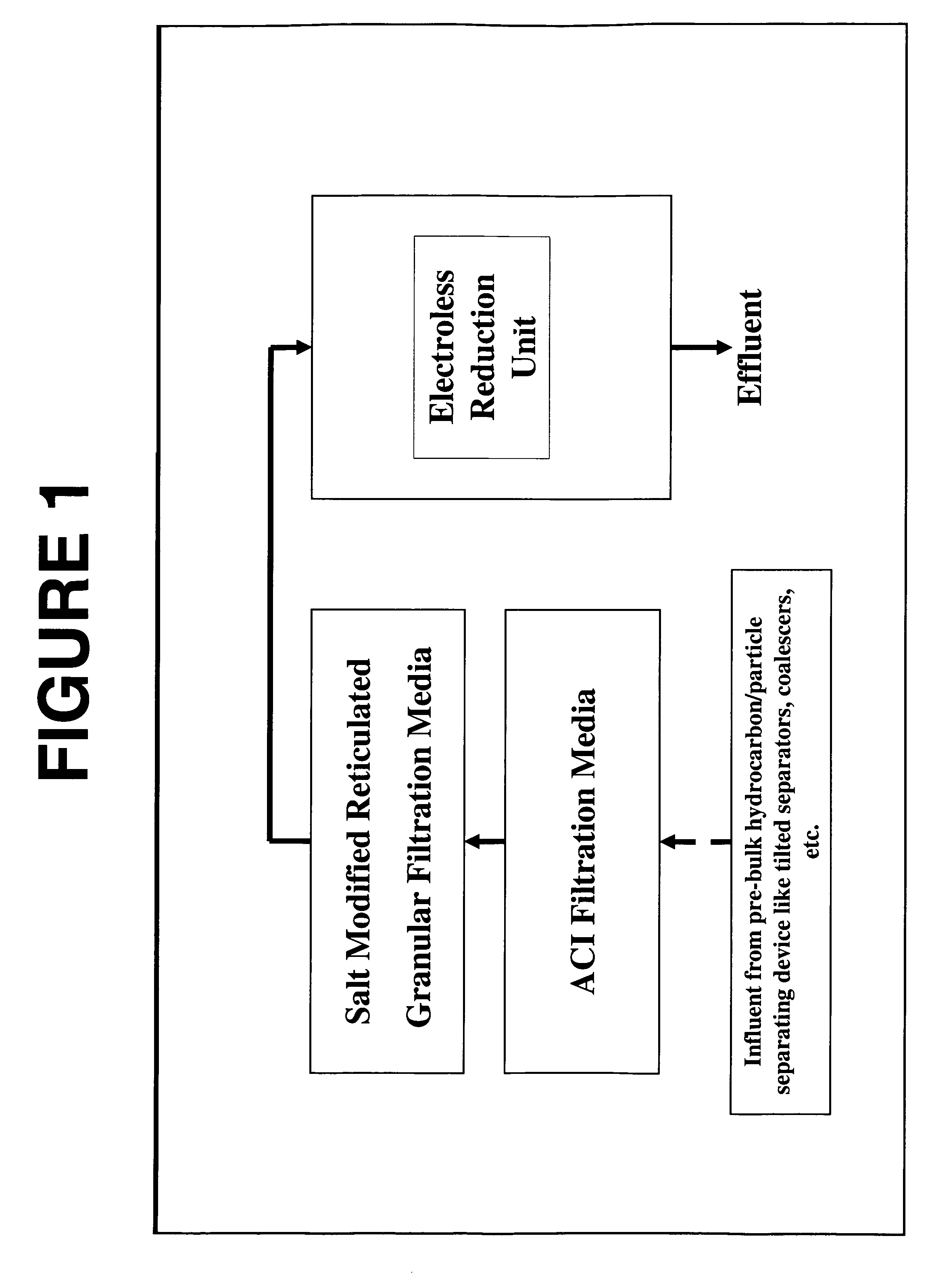
A method and system for removing from an aqueous system which is contaminated therewith: (1) mercury present as colloids, ions and / or organically bound compounds, and (2) hydrocarbons solubilized, dispersed, and / or emulsified in the said system. Pursuant to the invention the aqueous system to be treated (such as “produced water”) is passed successively through three filtration stages. The first filtration stage is provided with absorption media which effects reduction / removal of dispersed organically bound mercury species and of the dispersed and partially dissolved hydrocarbon phases, as well as of some colloidal mercury and other dissolved metallic species. The second filtration stage utilizes a salt modified reticulated granular filtration media for reduction / removal of slightly dissolved hydrocarbon phases, mercury in colloidal and ionic form and other dissolved metals. The third filtration stage is a polishing stage, which serves to further reduce by electroless or voltaic reduction residual elemental mercury and / or residual colloidal and ionic mercury. At this third stage metallic mercury is incorporated into a metallic matrix from which the mercury may preferably be recovered.
Owner:MYCELX TECH CORP
Rapid testing card for heavy metal mercury and testing method thereof
ActiveCN102776266BLow costSimple and fast operationMicrobiological testing/measurementPhysical chemistryEngineering
The invention discloses a rapid testing card for heavy metal mercury and a testing method thereof. The structure of the testing card includes a base board, absorption wafers and a cover film successively from bottom to top. At least one wafer for absorbing color-developing agents and at least one wafer for absorbing urease are respectively fixed on the base board, and the wafers are not overlapped mutually. The use amount ratio of the urease and the color-developing agents absorbed by the wafer per unit area is 3U: (1.6-3.2mL), and the color-developing agents contain components of, by mass, 0.05% to 2.5% of phenol red and 0.8% to 2% of urea. The testing card can test the content of mercury larger than or equal to 0.2 mg / L rapidly, accurately and conveniently; the test card is low in cost, simple and convenient to operate, short in testing time, in no need of professionals, sensitive in reaction and easy to observe and interpret; and the testing card is non-toxic and harmless and cannot cause environment pollution.
Owner:广东中检达元检测技术有限公司
Method and Device for Separation of Recoverable Material from Products Containing Mercury
Method of separating recoverable material from products containing mercury. The method including crushing products to form crushed material, mixing crushed material with a liquid which has an oxidizing agent which has been chosen from a group which sodium hypochlorite, hydrogen peroxide and chlorates, oxidizing at least a portion of metallic mercury in the products for forming mercury oxide under influence of oxidizing agent. The method further includes separating a sludge, which sludge having formed mercury oxide, from at least a portion of the liquid.
Owner:MIDAS INVESTMENTS
Method for recycling mercury from waste and old neutral zinc-manganese dioxide battery
InactiveCN102117919AHigh and stable recoveryReduce addReclaiming serviceable partsProcess efficiency improvementZinc ionManganese
The invention discloses a method for recycling mercury from a waste and old neutral zinc-manganese dioxide battery. The method comprises the following steps: taking out a cathodic zinc skin of a waste and old neutral zinc-manganese dioxide battery, then placing anodic substances and electrolyte of the waste and old neutral zinc-manganese dioxide battery into water, stirring to ensure that the anodic substances and the electrolyte are dispersed, adjusting the pH value to 1-6 with acid, sufficiently soaking and stirring to enable the electrolyte and mercury compounds in the waste and old neutral zinc-manganese dioxide battery to be dissolved in the water, filtering undissolved substances, and recycling; repeatedly using the filtrate to soak the anodic substances and the electrolyte, placingthe cathodic zinc skin into the filtrate when the concentration of the zinc ions in the filtrate reaches the weight percent of 0.1-10%, and stirring to enable the cathodic zinc skin to sufficiently contact the filtrate, thus enabling the mercury ions to replace zinc and generating zinc amalgam; when the mercury ions react completely, taking out the amalgamated zinc skin from the filtrate, and electrolyzing by taking the amalgamated zinc skin as an anode and taking the filtrate as the electrolyte; and when the zinc on the anode is dissolved and the metallic mercury is peeled off below the anode in the electrolyzing process, collecting the fallen metallic mercury, thus finishing the recycle of the mercury.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com