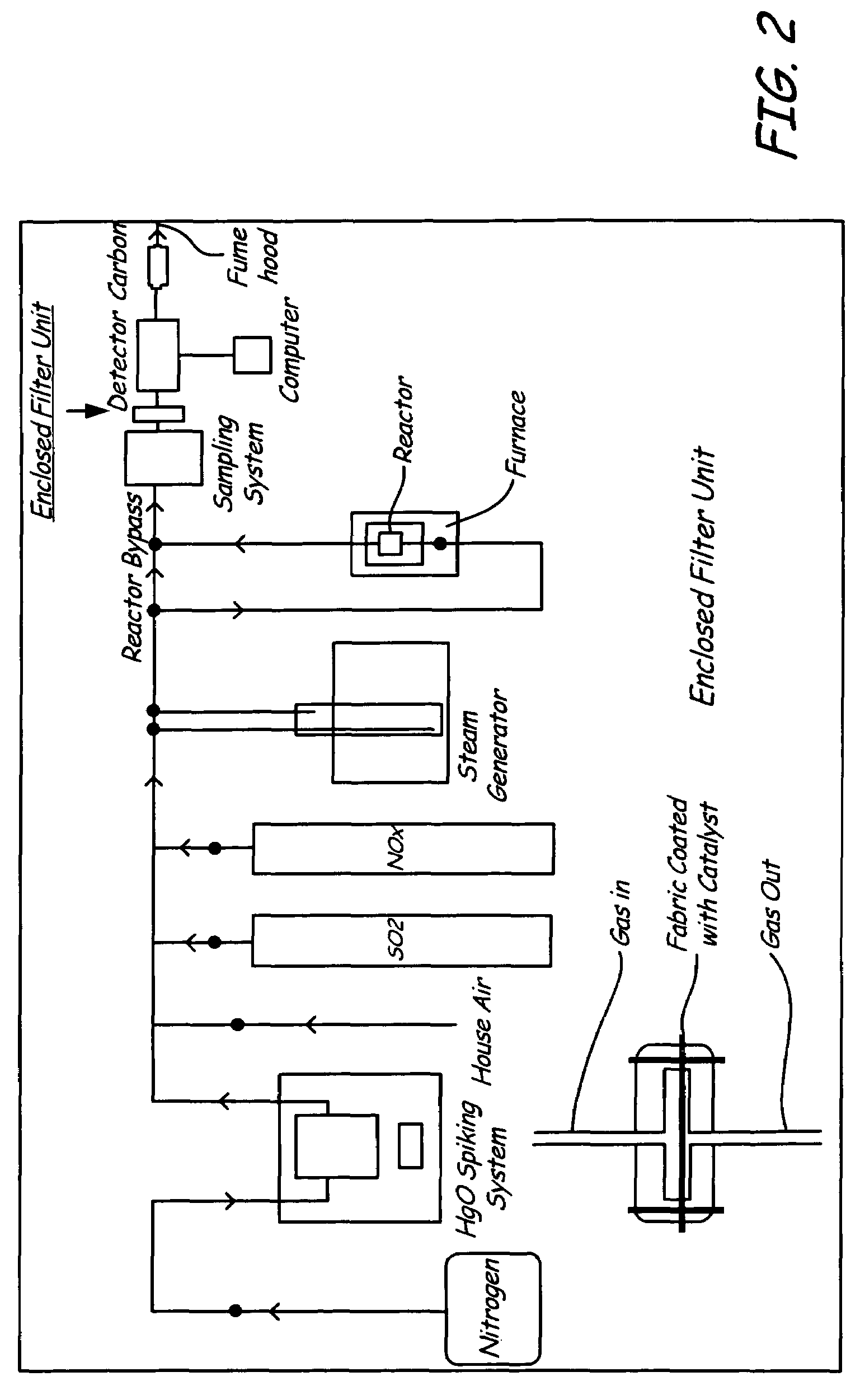
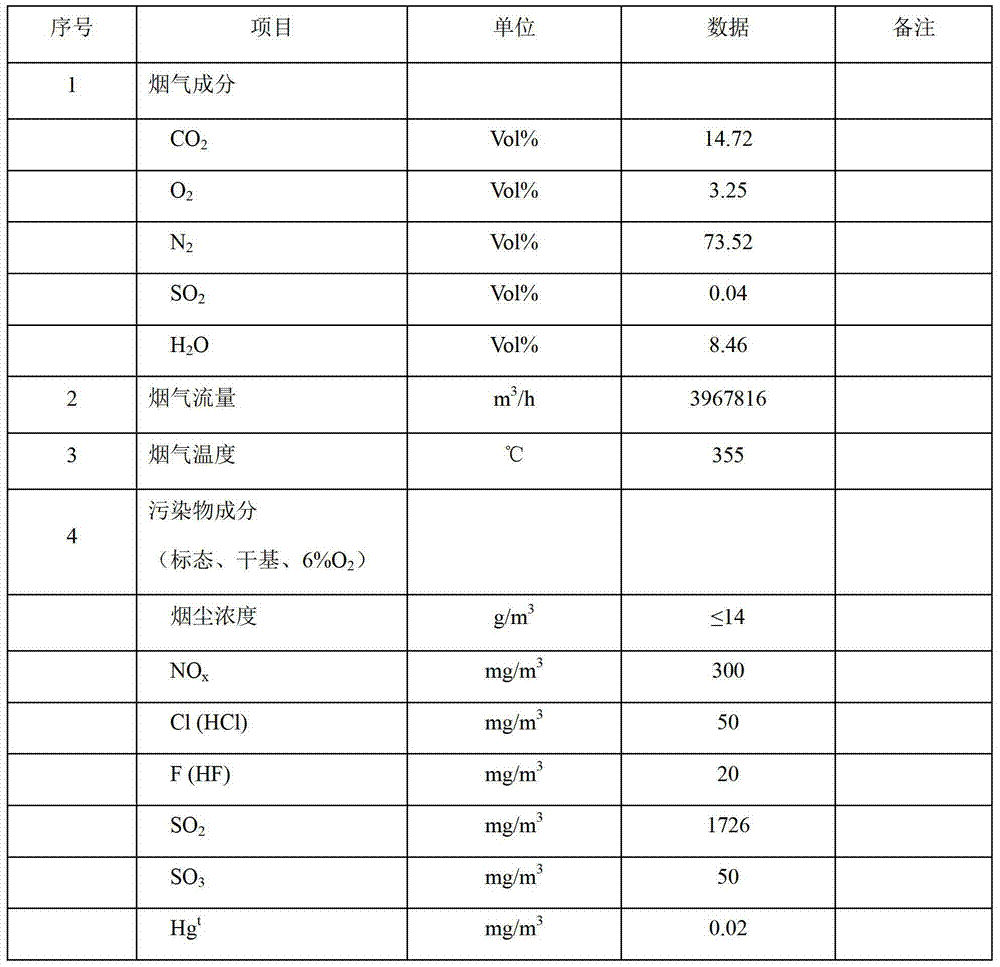
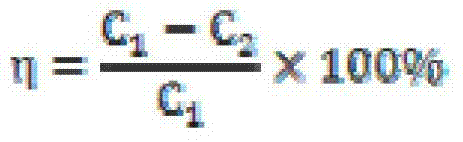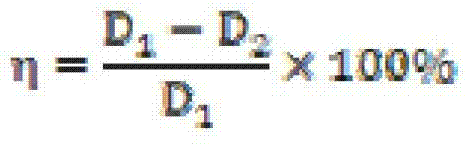Patents
Literature
87 results about "Mercury oxidation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In compounds of mercury (where known), the most common oxidation numbers of mercury are: 2, and 1.
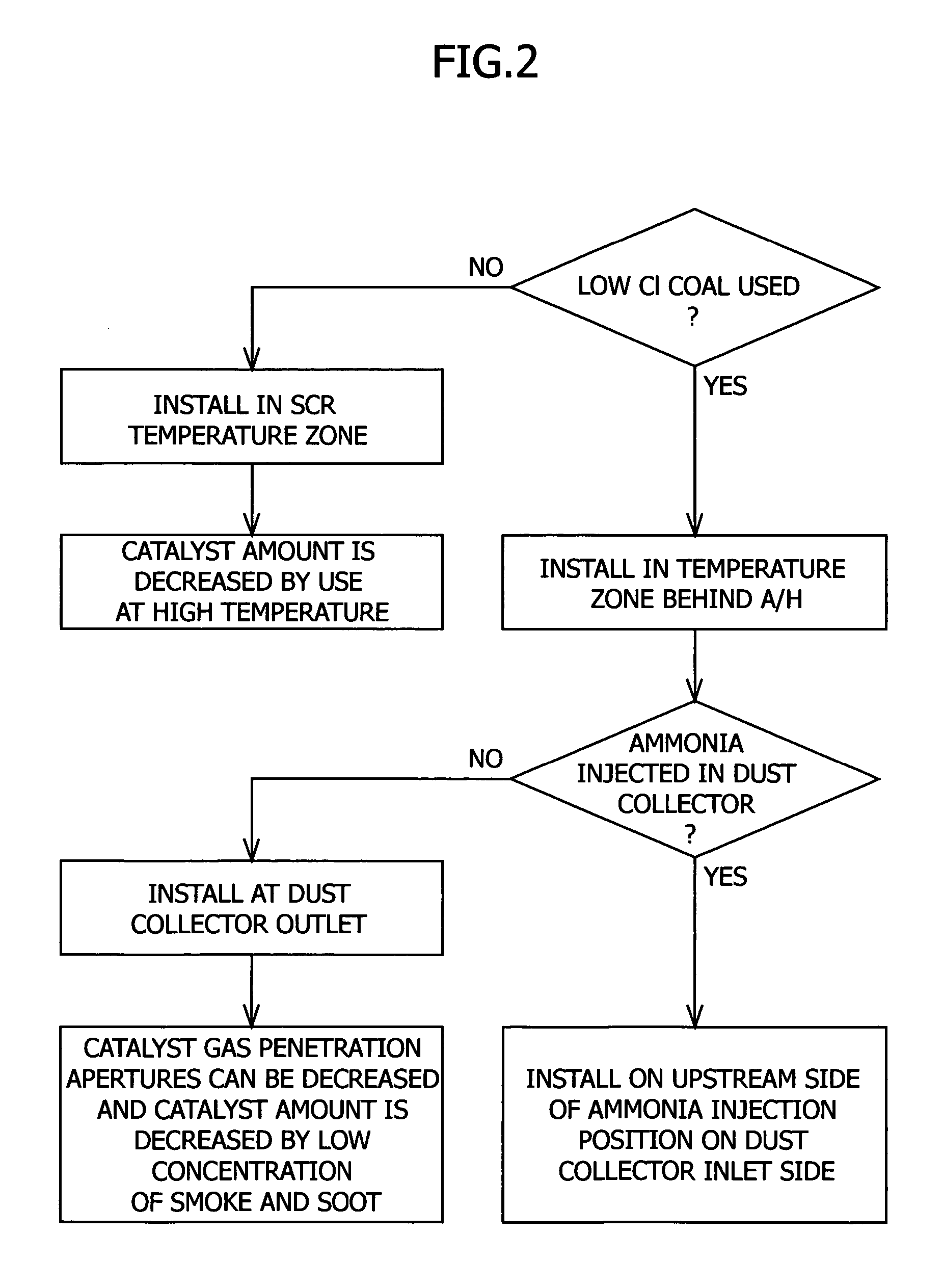
Method of removing mercury from flue gas through enhancement of high temperature oxidation
InactiveUS20060029531A1Increased formationIncrease volumeGas treatmentUsing liquid separation agentGas phaseWastewater
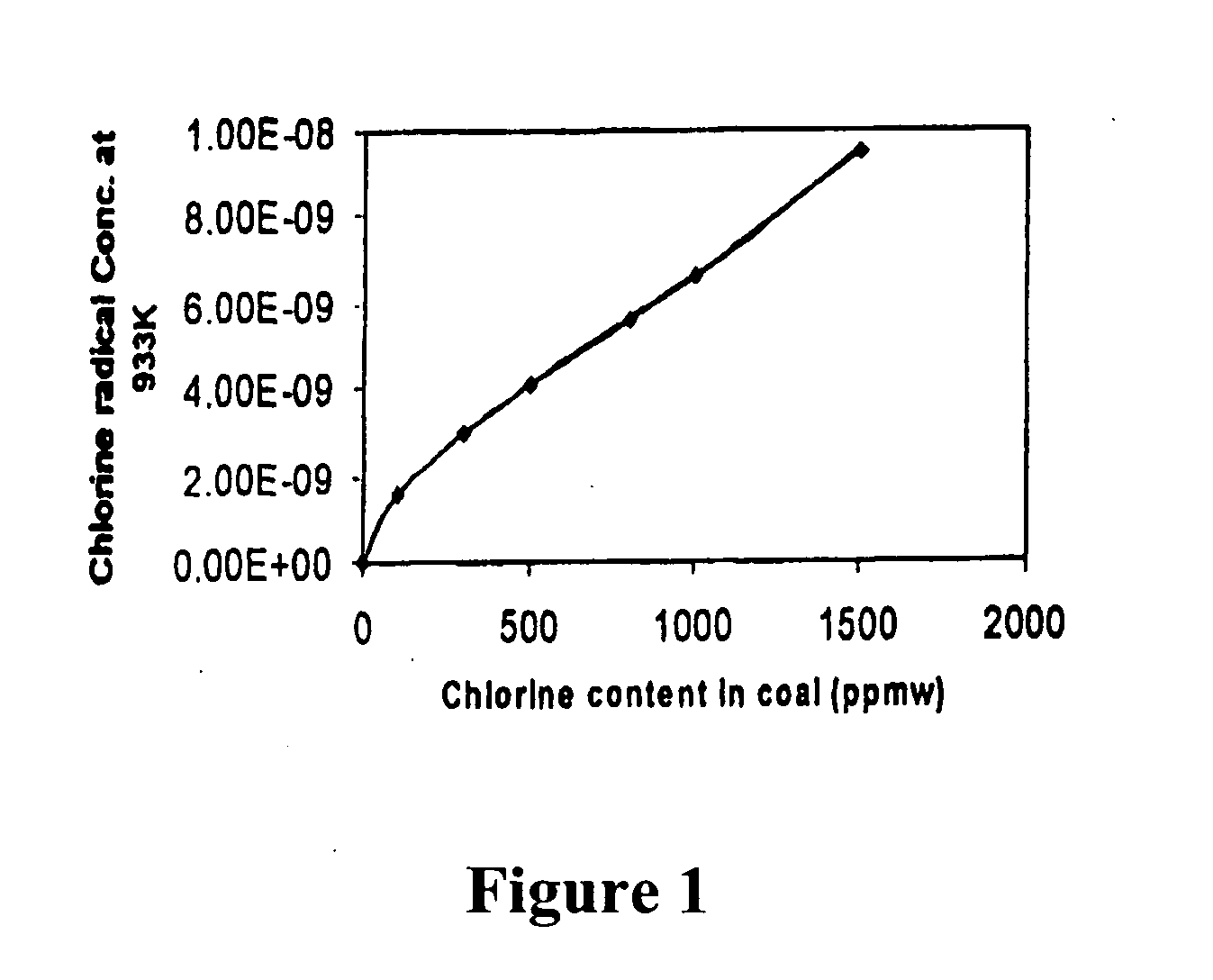
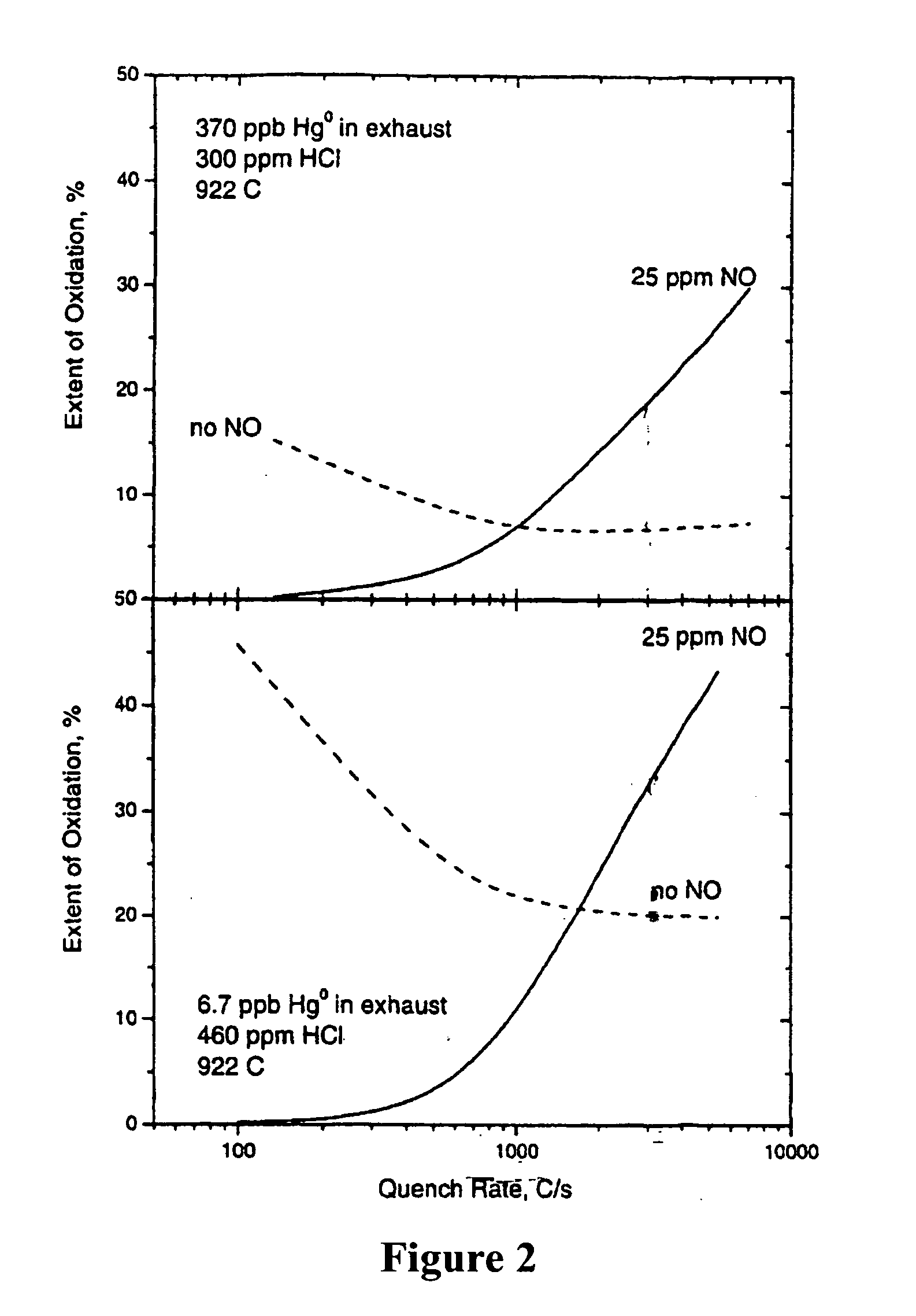
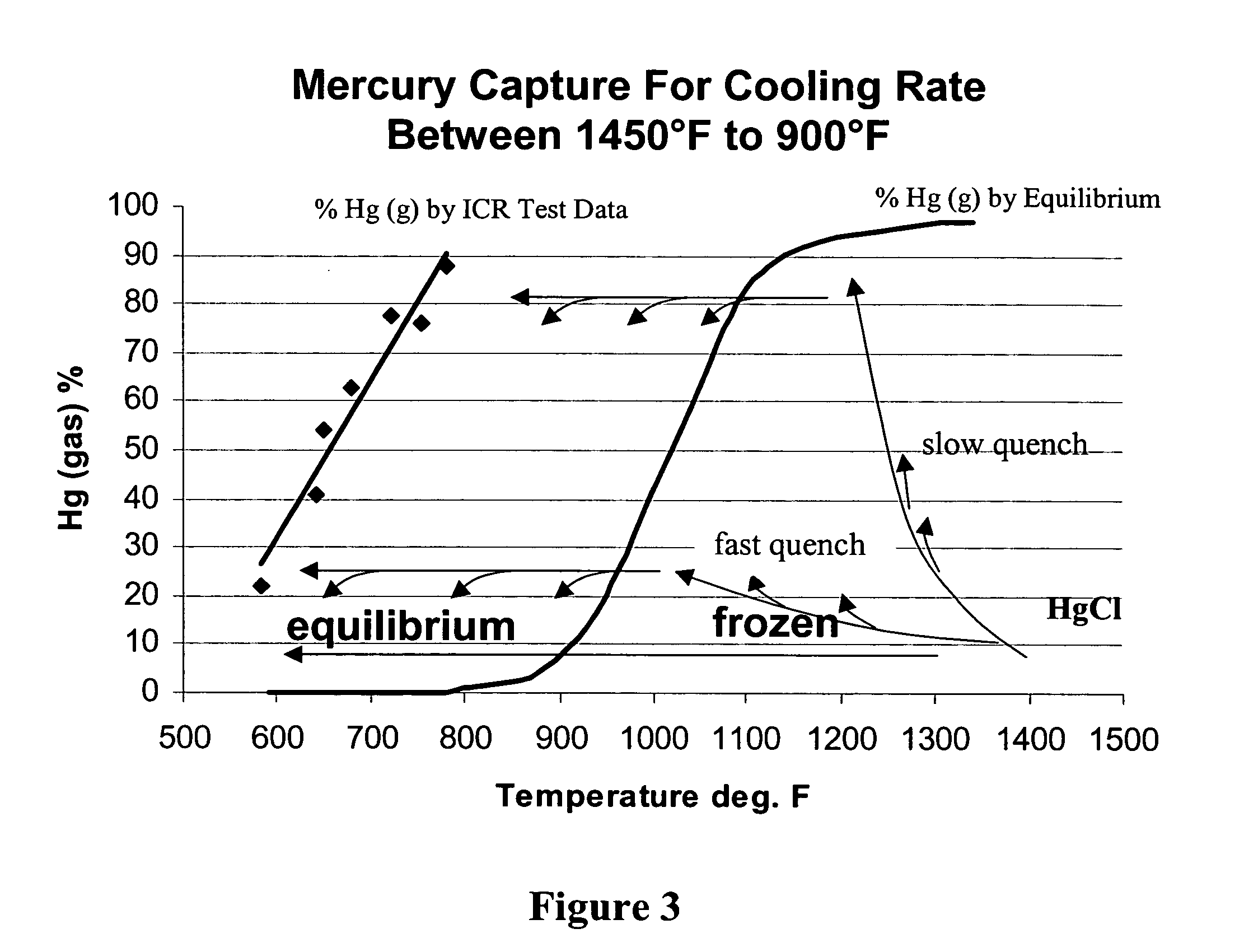
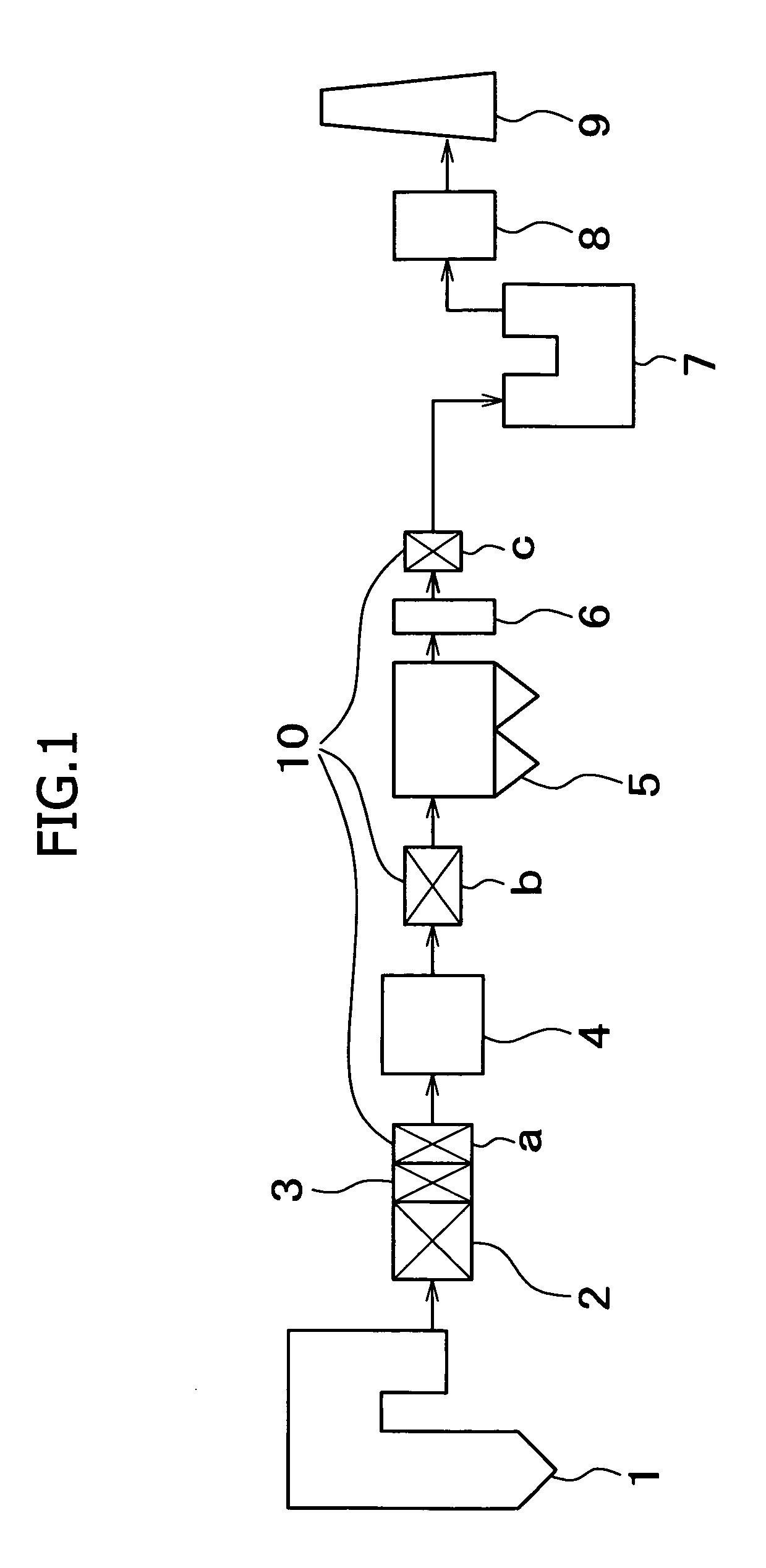
In a method for removing mercury from flue gas produced by combustion devices burning coal and other fuels that contain mercury and chlorine the combustion process is controlled to generate a flue gas comprising fly ash containing at least 0.25% unburned carbon, and preferably at least 5.0% unburned carbon. In addition the flue gas is rapidly cooled from a temperature within the range of 1450° F. to 900° F. to a temperature below 900° F. at a rate of at least 1000° F. per second. This step will enhance the concentrations of Cl-atoms and Cl2, which accelerates the rates of mercury oxidation in both the gas phase and on particle surfaces. Finally, the flue gas is directed to the particle removal device for removal of the fly ash to which some of the mercury is bound, and also directed to a wet scrubber for retention of the oxidized mercury vapor in wastewater and solid effluents.
Owner:BREEN ENERGY SOLUTIONS
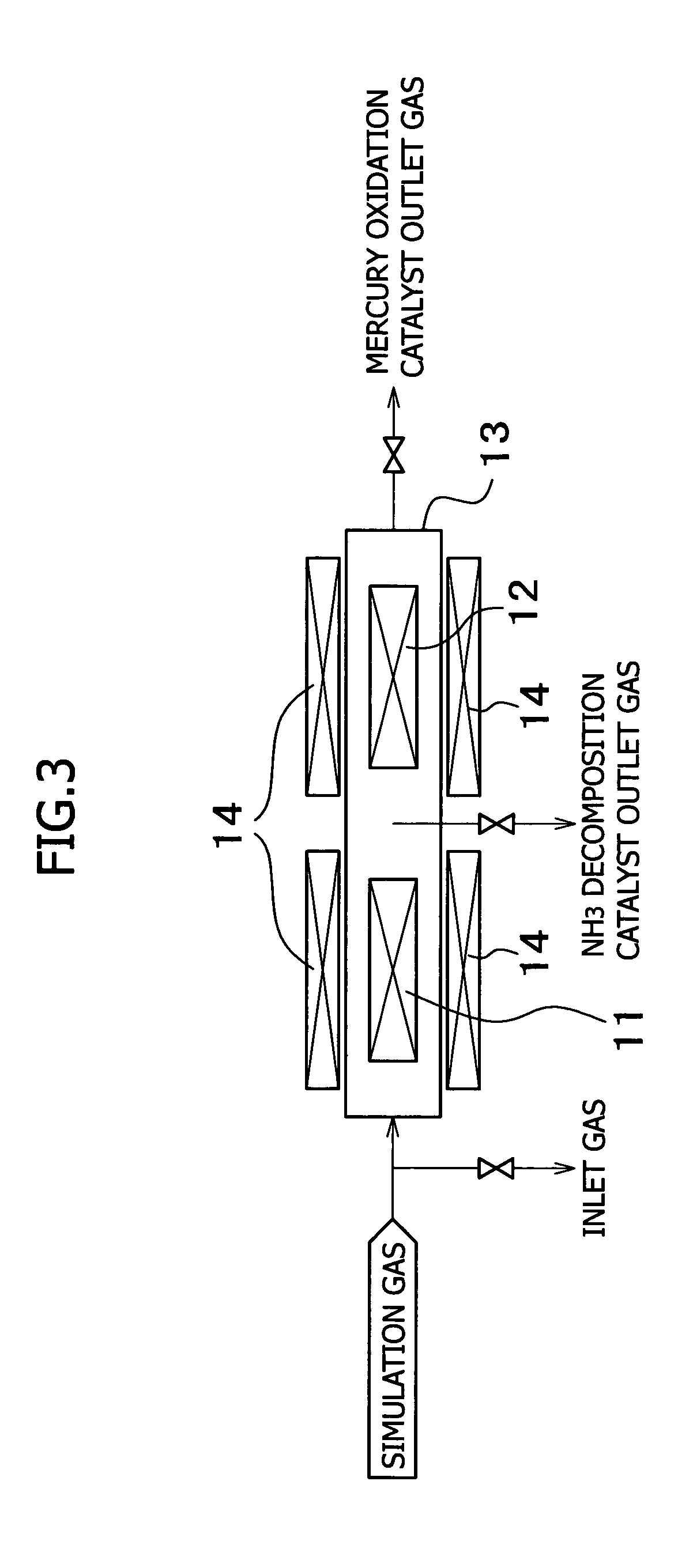
Method for removing mercury in exhaust gas
InactiveUS7521032B2Amount of catalyst can be reducedIncreased durabilityCombination devicesGas treatmentDecompositionExhaust fumes
The present invention provides a system for removing mercury in exhaust gas, in which mercury is removed from exhaust gas of a boiler, characterized in that between a denitrification apparatus and a wet type desulfurization apparatus, an NH3 decomposition catalyst and a mercury oxidation catalyst are provided, and mercury having been oxidized into mercury chloride is removed by the wet type desulfurization apparatus. Also, it provides a method for removing mercury in exhaust gas, characterized in that the mercury removing method includes an NH3 decomposition process and a mercury oxidation process, which are provided between the denitrification process and a wet desulfurization process, and mercury having been oxidized into mercury chloride is removed in the wet desulfurization process.
Owner:MITSUBISHI HITACHIPOWER SYST LTD
Flue gas hydrargyrum-removing method by catalytic oxidation
InactiveCN1698931ALittle matrix damageReduce pollutionDispersed particle separationPtru catalystSorbent
This invention relates to a catalytic oxidation smoke gas mercury removing method, which is used for controlling and reclaiming the gas mercury, especially for the brown coal and subbituminous coal smoke gas. The method uses the mercury oxidative catalyst and the adsorbent to oxygenize and absorb mercury in the smoke gas, then dissociate and elution the mercuric oxide in high temperature to regenerate it. The heat of the elution gas can be reclaimed by residual heat utilization device, and the high concentration mercury will be reclaimed through cold-trap or processed by the agent in the mercury reactor. The reclaimed mercury and other by-product can be sold to reduce the cost. Said invention uses the thermal instability of the mercuric oxide, and reclaims in the relative lower temperature; the recovery ratio can reach to 98%, meanwhile, it processes post-treatment with the mercury and has no secondary pollution.
Owner:SHANGHAI JIAO TONG UNIV
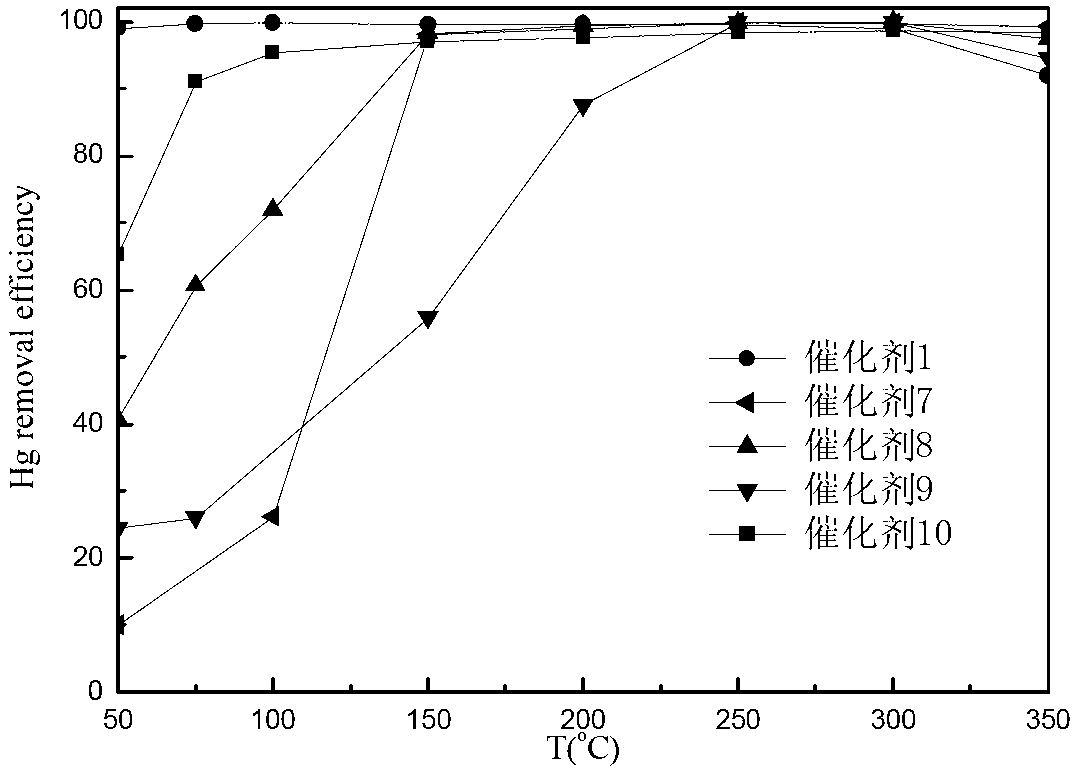
Catalyst for mercury oxidation and preparation method and purpose thereof
ActiveCN102698753AReduce dependenceImprove oxidation efficiencyDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsPower stationCatalytic oxidation
The invention discloses a copper-based composite catalyst for gas-state zero valent mercury oxidation in the field of smoke control and a preparation method and the purpose thereof. The copper-based catalyst is a copper-based composite oxide catalyst and / or a copper-based composite halide catalyst. The catalyst can utilize oxygen and trace HCI existing in smoke to achieve high efficiency conversion of zero valent mercury (HgO) and divalent mercury (Hg2+) under low temperature in a wider temperature range (500-300 DEG C) and can even achieve high efficiency oxidation of simple substance mercury in smoke free of HCI. The catalyst is suitable for catalytic oxidation of mercury in fire coal smoke, and the product Hg2+ of oxidation can be easily dissolved in water and can be removed by other pollutant control devices. The catalyst is simple in preparation method, wide in suitable temperature range, high in oxidation efficiency, weak in dependency on CI in the smoke, good in stability and high in SO2 poisoning resistance and has good application prospects and economical benefits in the fire coal smoke mercury discharge control fields including power station boilers, industrial boilers, industrial kilns and the like.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Catalyst for oxidation of metal mercury
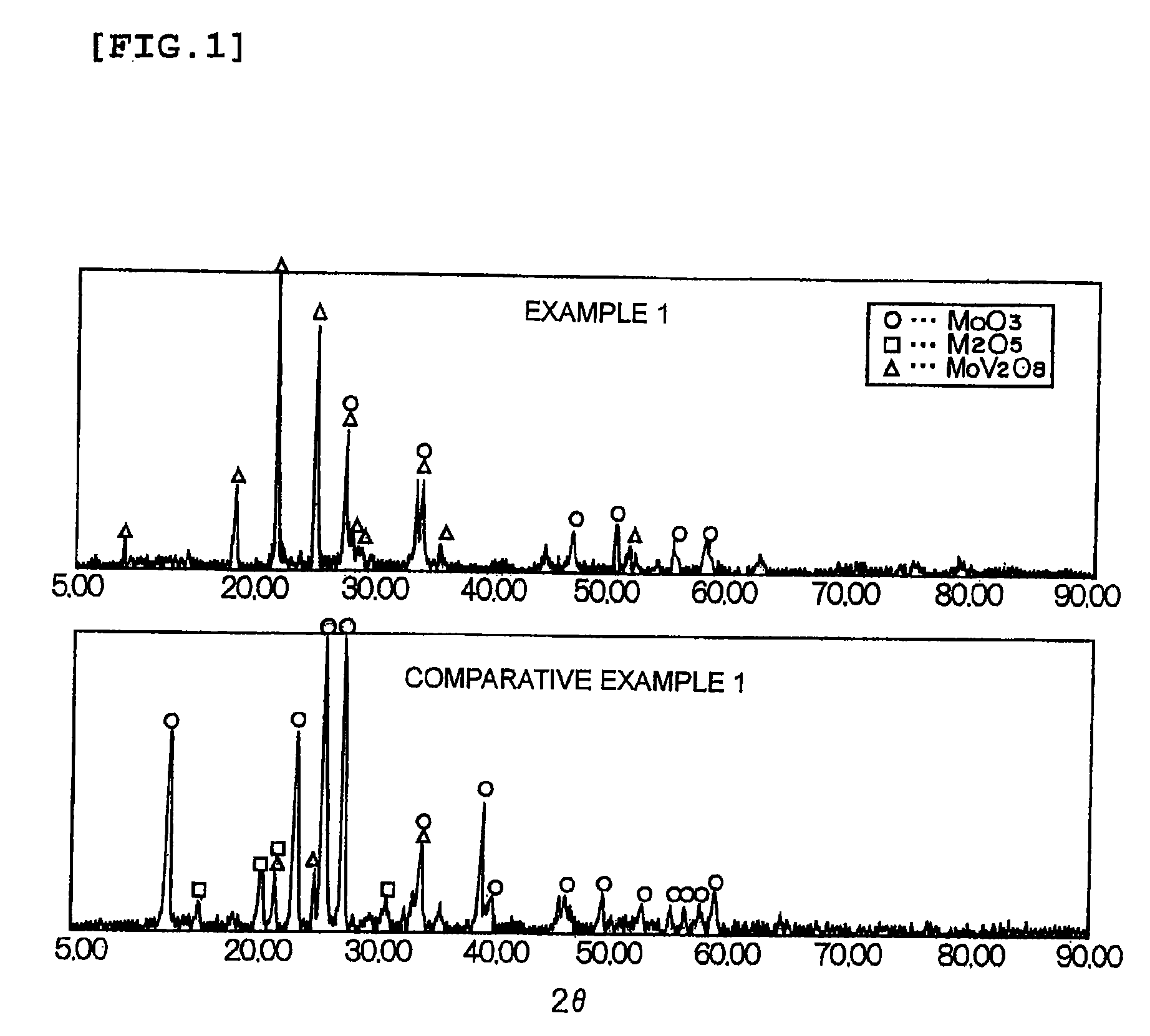
ActiveUS7842644B2Efficiently oxidizedImprove performanceDispersed particle filtrationElectrostatic separationHoneycomb likeMercury oxidation
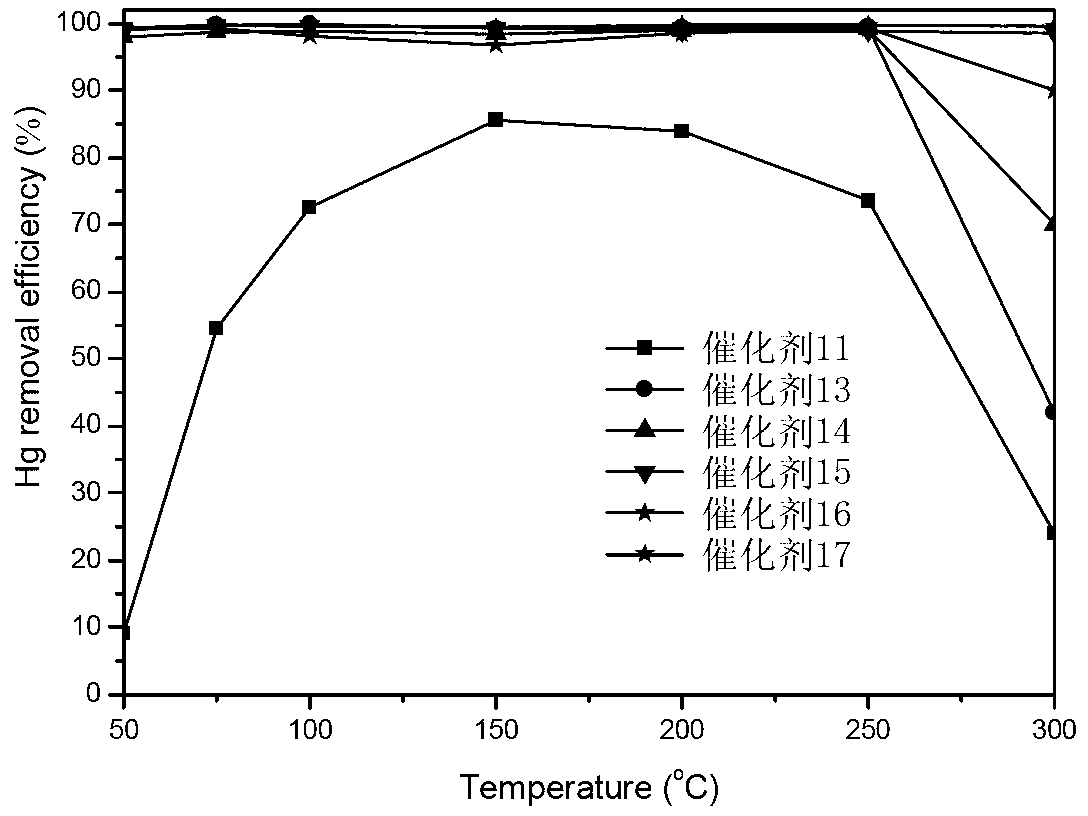
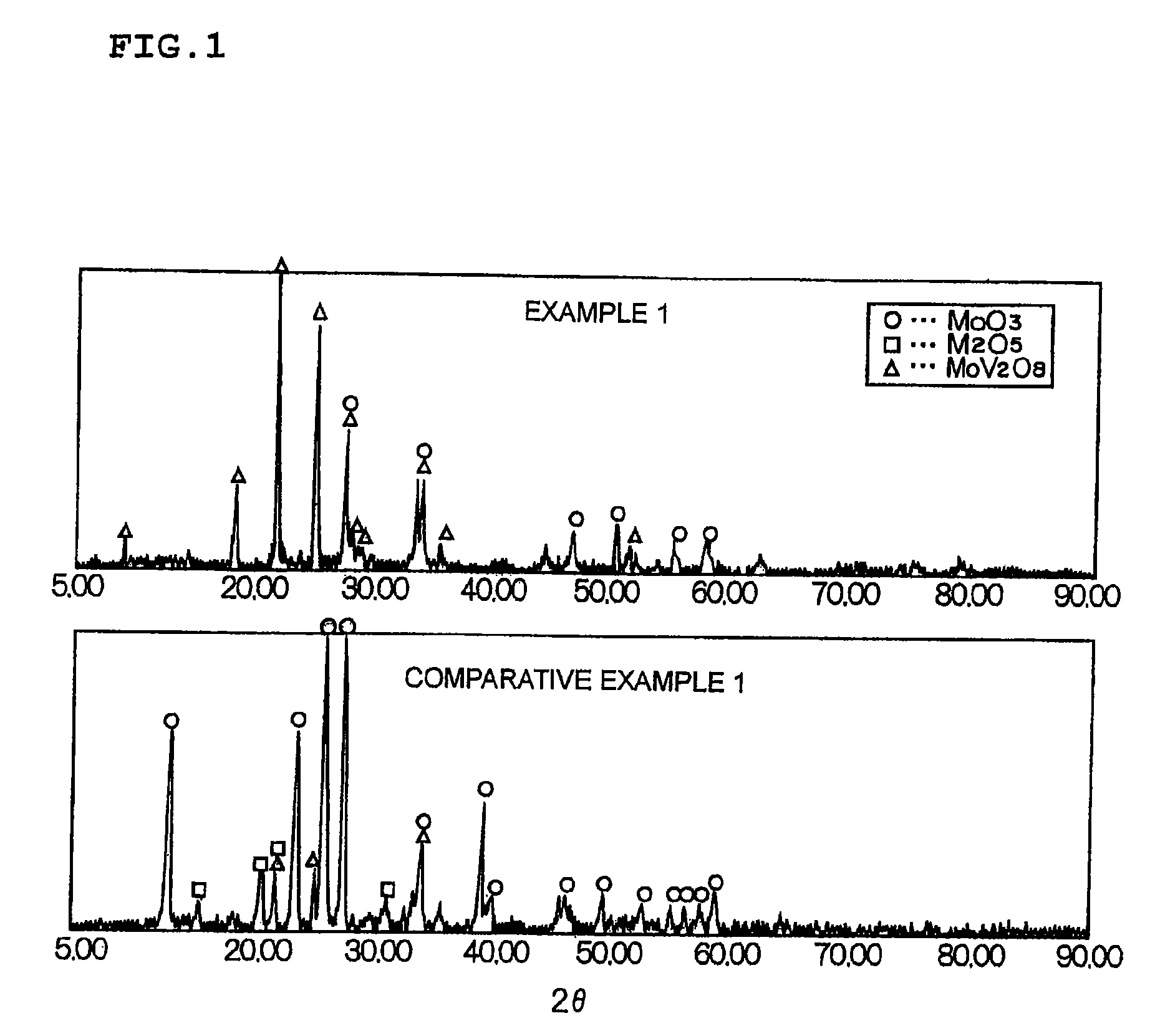
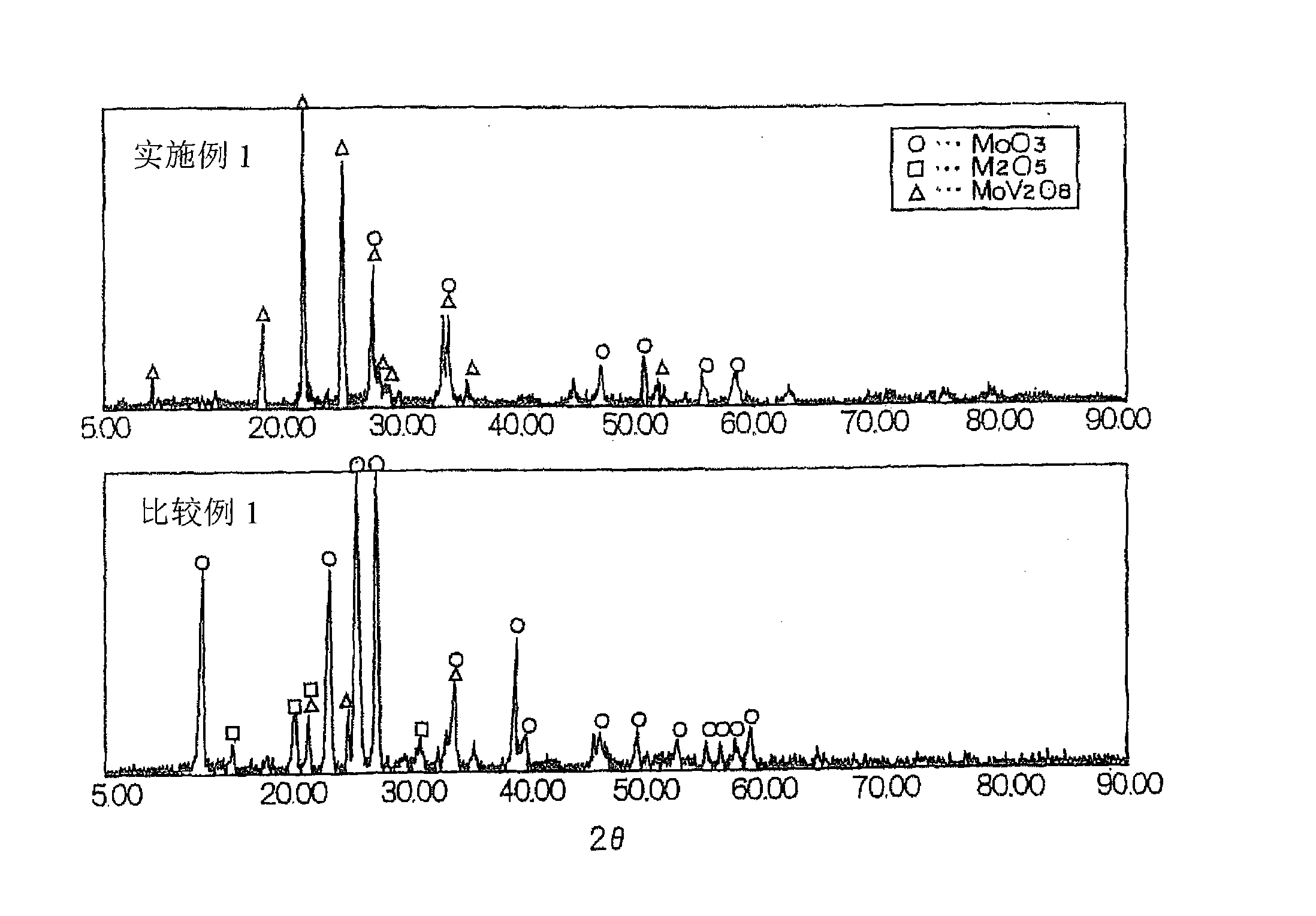
A catalyst is provided having higher mercury oxidation performance than a conventional catalyst without increasing catalyst quantity or enhancing SO2 oxidation performance and constitutes an oxidation catalyst for metal mercury, which contains a molybdenum and vanadium complex oxide, for example, MoV2O8, as a main component having a catalytic activity and is formed by placing the molybdenum and vanadium complex oxide in layers only on the surface of a plate-like or honeycomb-like porous carrier. The porous carrier contains Ti and W and has a function of an NOx removal catalyst as a whole.
Owner:MITSUBISHI POWER LTD
Mercury oxidation of flue gas using catalytic barrier filters
InactiveUS7618603B2Facilitates simultaneous removalCombination devicesGas treatmentOxidizing agentMercury oxidation
A method for oxidizing elemental mercury contained in flue gas uses a catalytic barrier filter. The method comprises directing the flue gas towards the catalytic barrier filter; passing the flue gas through the catalytic barrier filter in the presence of an oxidant; and outletting the flue gas from the catalytic barrier filter, wherein about 50 percent to about 99 percent of the elemental mercury is oxidized.
Owner:NORTH DAKOTA THE UNIV OF
Flue gas mercury absorption liquid with functions of oxidizing and fixing synchronously
The invention discloses flue gas mercury absorption liquid with functions of oxidizing and fixing, which consists of calcium-based desulphurization slurry, Fenton reagent and soluble thiosulfate. The Fenton reagent is used as oxidant to oxidize zero-state mercury, and the thiosulfate is used as a stabilizing agent to precipitate and stabilize the absorbed mercury. The precipitated mercury and the generated gypsum are precipitated together to form a solid phase, a part of the absorbed sulfur dioxide is absorbed into a liquid phase and oxidized into gypsum under the action of redundant oxidant, the absorption liquid separates the gypsum from the liquid phase through solid-liquid separation modes such as precipitation and the like, and the mercury is separated and removed at the same time. The mercury absorption liquid of the invention can be used for flue gas purification, and has the capacities of mercury oxidation absorption and liquid phase stabilization.
Owner:ZHEJIANG TIANLAN ENVIRONMENTAL PROTECTION TECH
Denitration and demercuration catalyst as well as preparing method and application thereof
InactiveCN103480371AGood dispersionImprove removal efficiencyDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsPtru catalystRuthenium
The invention provides a denitration and demercuration catalyst as well as a preparing method and application thereof. The method includes the following steps: mixing and contacting a ruthenium-containing substance, a ti-based denitration catalyst with hydrogen peroxide; roasting the contacted mixture dried or undried. According to the invention, as the ruthenium-containing substance, the ti-based denitration catalyst and the hydrogen peroxide are mixed and contacted, and the contacted mixture dried or undried is roasted, the catalyst obtained through adopting the method has the functions of denitration and demercuration, obviously improves the NOx removal efficiency compared with the ti-based denitration catalyst with simple function, and is very high in oxygenation efficiency of Hg<0>(zero valent mercury), namely, the demercuration efficiency is very high.
Owner:CHINA SHENHUA ENERGY CO LTD +2
Filter for Removal of Heavy Metals
A filter contains a textile having on at least one side a plurality of treated activated carbon particles. The treated activated carbon particles contain a plurality of activated carbon particles, a hydrophobic agent, a mercury oxidation facilitation agent, and optionally a binder.
Owner:MILLIKEN & CO
Method and device for collaborative removal of mercury in flue gas
InactiveCN102989282AControl concentrationControl/or quantityDispersed particle separationDust controlEnvironmental engineering
The invention discloses a method and device for the collaborative removal of mercury in flue gas, and belongs to the purification field of the flue gas in atmospheric pollutants of power plant boilers. The method comprises the following steps that: after primary hot air or secondary hot air of a temperature boiler is introduced to mix with a mercury removal oxidant and an SCR (selective catalytic reduction) denitration reductant, and then the flue gas is injected to mix again, an obtained mixture enters an SCR denitration device to denitrate along a flow path of the flue gas, and simultaneously, elemental mercury Hg0 is oxidated into gaseous bivalent mercury oxide Hg<2+>; then, the gaseous bivalent mercury oxide Hg<2+> enters a dust remover to carry out dust removal, and simultaneously, granular mercury Hg is removed; and then, the obtained product is subjected to desulfurization in a wet flue gas desulfurization device, and simultaneously, the bivalent mercury oxide Hg<2+> is removed. The device comprises an SCR denitration system, and a device for mixing hot air, a mercury removal oxidant and the SCR denitration reductant. The method and the device for the collaborative removal of the mercury in the flue gas, disclosed by the invention, fully use the collaborative function of the existing SCR denitration system, the dust remover and the WFGD (wet flue gas desulfurization device), and are few in system changes and good in mercury removal effect.
Owner:DONGFANG BOILER GROUP OF DONGFANG ELECTRIC CORP
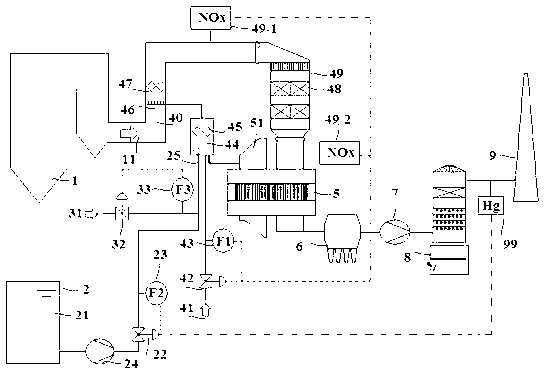
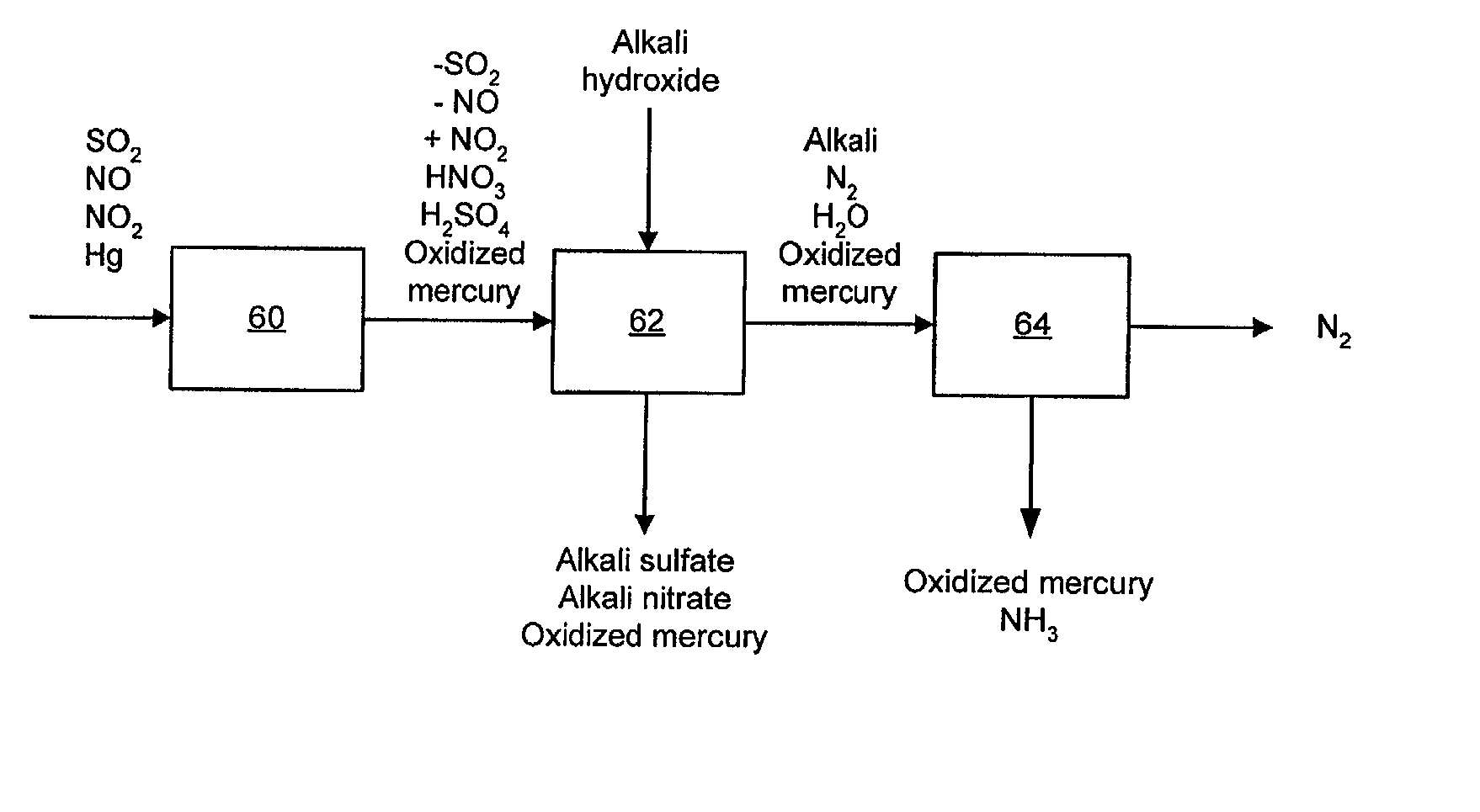
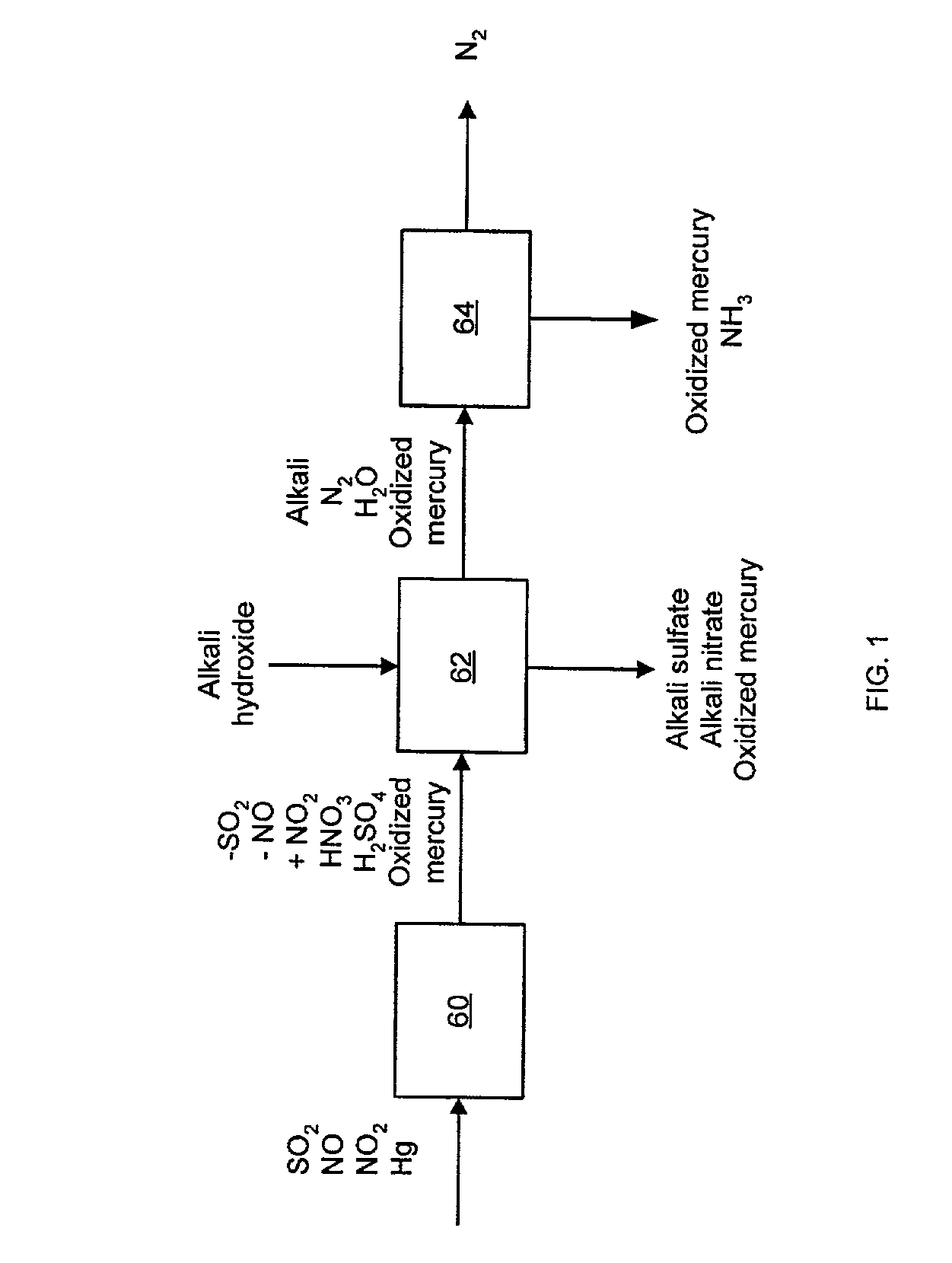
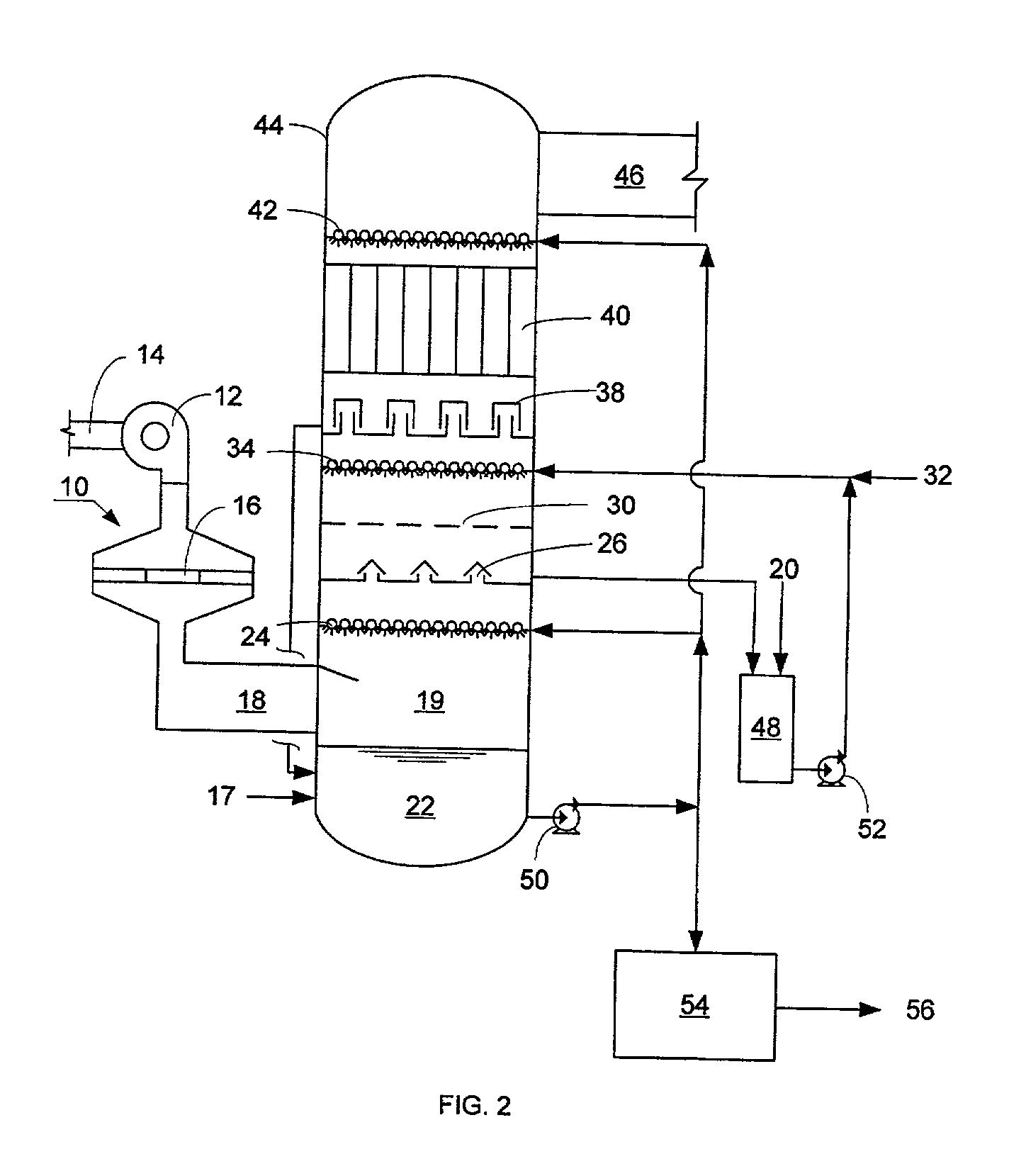
NOx, Hg, and SO2 removal using alkali hydroxide
A process and apparatus for removing SO2, NO, and NO2 from a gas stream having the steps of oxidizing a portion of the NO in the flue gas stream to NO2, scrubbing the SO2, NO, and NO2 with an alkali scrubbing solution, and removing any alkali aerosols generated by the scrubbing in a wet electrostatic precipitator. The process can also remove Hg by oxidizing it to oxidized mercury and removing it in the scrubbing solution and wet electrostatic precipitator. Alkali sulfates, which are valuable fertilizers, can be withdrawn from the rubbing solution.
Owner:POWERSPAN CORP
Catalyst for oxidation of metal mercury
ActiveCN101528343AImprove wear resistanceHeterogenous catalyst chemical elementsDispersed particle separationHoneycomb likeMercury oxidation
The invention is directed to providing a catalyst having higher mercury oxidation performance than a conventional catalyst without increasing catalyst quantity or enhancing SO 2 oxidation performance and constitutes an oxidation catalyst for metal mercury, which contains a molybdenum and vanadium complex oxide, for example, MoV 2 O 8 , as a main component having a catalytic activity and which is formed by placing the molybdenum and vanadium complex oxide in layers only on the surface of a plate-like or honeycomb-like porous carrier. The porous carrier contains Ti and W and has a function of an NOx removal catalyst as a whole.
Owner:MITSUBISHI POWER LTD
Novel demercuration catalyst
InactiveCN102764655AImprove mercury removal efficiencyLow costDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsBiological bodyMercury adsorption
Disclosed is a novel demercuration catalyst. As mercury has extremely high accumulation performance in the environment and in organisms, it has very strong toxicity for mankind and wild animals and plants even the concentration is very low. In recent years, mercury is considered as another pollution problem in the world. Present existing demercuration catalysts have disadvantages of poor anti-poisoning capability and high cost, and are difficult to realize industrialization. In order overcome these advantages, a novel demercuration catalyst is invented. The product is prepared from TOx (T is Cu, Fe and V) and ROy (R is La and Ce) with the addition of titanium dioxide pillared montmorillonite. The invention belongs to the technical field of separation. The demercuration catalyst can be used for elemental mercury removal from flue gas. The invention is characterized in that the catalyst has high mercury adsorption capability and mercury oxidation catalysis capability. The catalyst has very strong anti-poisoning capability.
Owner:YANCHENG INST OF TECH
Sorbent comprising carbon and nitrogen and methods of using the same
Owner:MIDWEST ENERGY EMISSIONS CORP
Method for removing mercury in exhaust gas and system therefor
The present invention provides a method for removing mercury in exhaust gas, in which mercury in exhaust gas discharged from combustion equipment is removed, characterized by including a mercury oxidation process in which mercury in the exhaust gas is converted to mercury chloride in the presence of a catalyst; a contact process in which the exhaust gas is brought into contact with an absorbing solution in a scrubber to absorb and remove mercury components from the exhaust gas; and a control process in which blowing of air or addition of an oxidizing agent into the scrubber is accomplished, and the amount of blown air or the added amount of oxidizing agent is regulated to control the oxidation-reduction potential of the absorbing agent, and a system for removing mercury in exhaust gas. According to the mercury removing method in accordance with the present invention, a phenomenon that mercury chloride is reduced into metallic mercury by SO2 etc. and the metallic mercury scatters in the exhaust gas can be prevented, and mercury in the exhaust gas can be decreased effectively.
Owner:MITSUBISHI POWER LTD
Exhaust gas treatment system and a method of treating exhaust gas
InactiveUS20140079615A1Reduce NOxReduce operating costsCombination devicesGas treatmentMercury oxidationAmmonium sulfate
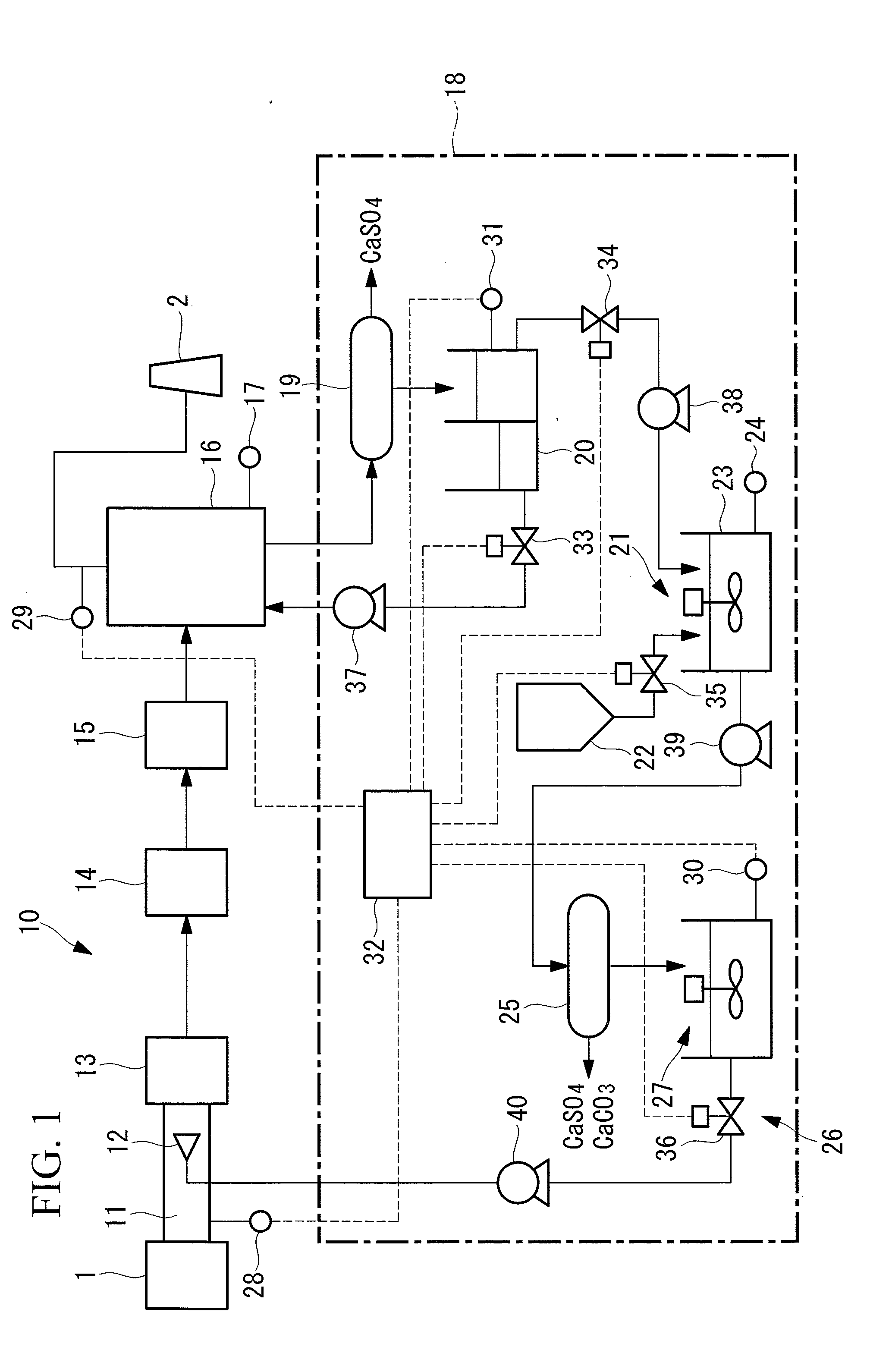
A flue gas treatment system and a flue gas treatment method capable of drastically reducing operating cost and an amount of waste water and capable of controlling an amount of a NOx reducing agent and a mercury oxidation agent to be supplied are provided. The flue gas treatment system sprays an aqueous solution containing ammonium halide into a flue, reduces NOx and oxidizes mercury in a deNOx section, and removes SOx and the mercury in a desulfurization section. The flue gas treatment system adds at least one of ammonium sulfate and ammonium carbonate to waste water which is discharged from the desulfurization section and from which CaSO4 is separated to generate the ammonium halide. The waste water containing the ammonium halide is sprayed as the aqueous solution.
Owner:MITSUBISHI HEAVY IND LTD
Exhaust gas purifying catalyst
ActiveUS8535628B2Improve performanceImprove oxidation activityCyanogen compoundsNitrogen compoundsPtru catalystNitrogen oxides
To overcome the problem of a conventional catalyst and to provide an exhaust gas purifying catalyst that meets the requirement concerning Hg oxidation activity and SO2 oxidation activity; i.e., an exhaust gas purifying catalyst which specifically reduces percent SO2 oxidation, while maintaining percent Hg oxidation at a high level.The invention provides an exhaust gas purifying catalyst which comprises a composition containing oxides of (i) titanium (Ti), (ii) molybdenum (Mo) and / or tungsten (W), (iii) vanadium (V), and (iv) phosphorus (P), wherein the catalyst contains Ti, Mo and / or W, and V in atomic proportions of 85 to 97.5:2 to 10: 0.5 to 10, and has an atomic ratio of P / (sum of V and Mo and / or W) of 0.5 to 1.5, and an exhaust gas purifying method comprising exposing an exhaust gas containing a nitrogen oxide (NOX) and metallic mercury (Hg) to the catalyst in the presence of ammonia as a reducing agent, to thereby perform reduction of NOX contained in the exhaust gas and oxidation of metallic mercury (Hg) contained in the exhaust gas.
Owner:MITSUBISHI POWER LTD
Method for oxidizing elemental mercury by improving gas selective catalytic reduction (SCR) denitration processing system
InactiveCN105289278AAvoid the effects of oxidationHigh removal rateDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsDischarge efficiencyNitrate
The invention discloses a method for oxidizing elemental mercury by improving a gas selective catalytic reduction (SCR) denitration processing system, wherein a traditional SCR catalyst layer is distributed in front of a mercury oxidation catalyst, and 1-2 layers of modified SCR catalysts are further distributed. A method for preparing a modified SCR catalyst is that predecessors of V2O5 and WO3 are added in the preparation of a SCR catalyst of V2O5-WO3 / TiO2, and cerous nitrate, cupric nitrate or cobalt nitrate is further added at the same time, and the oxidation efficiency of mercury in gas is above 80%. The method for oxidizing the elemental mercury by improving the gas SCR denitration processing system has the advantages that the method for oxidizing the elemental mercury by improving the gas SCR denitration processing system does not need to improve equipment based on an original gas SCR denitration processing system, does not need to use additional additives, does not need to be further provided with mercury removal equipment, and achieves to effectively denitration and mercury oxidation by utilizing the SCR catalyst and the modified SCR catalyst. Simultaneously, the method for oxidizing the elemental mercury by improving the gas SCR denitration processing system prevents the influence of NH3 to mercury oxidation by reasonably arranging the SCR catalyst and the modified SCR catalyst, thereby improving mercury discharge efficiency.
Owner:HEBEI UNIV OF TECH
Mercury oxidation of flue gas using catalytic barrier filters
InactiveUS20060159605A1Facilitates simultaneous removalCombination devicesGas treatmentMercury oxidationOxidizing agent
Owner:NORTH DAKOTA THE UNIV OF
Flue gas hydrargyrum-removing method by catalytic oxidation
InactiveCN1331571CLittle matrix damageReduce pollutionDispersed particle separationPtru catalystSorbent
Owner:SHANGHAI JIAO TONG UNIV
Catalyst for oxidation of metal mercury
ActiveUS20090311155A1Efficiently oxidizedImprove performanceDispersed particle filtrationElectrostatic separationHoneycomb likeMercury oxidation
A catalyst is provided having higher mercury oxidation performance than a conventional catalyst without increasing catalyst quantity or enhancing SO2 oxidation performance and constitutes an oxidation catalyst for metal mercury, which contains a molybdenum and vanadium complex oxide, for example, MoV2O8, as a main component having a catalytic activity and is formed by placing the molybdenum and vanadium complex oxide in layers only on the surface of a plate-like or honeycomb-like porous carrier. The porous carrier contains Ti and W and has a function of an NOx removal catalyst as a whole.
Owner:MITSUBISHI POWER LTD
Catalyst for synergistic control of oxynitride and mercury and method for preparing the same
ActiveUS20160354766A1Poor anti-poisoning abilityAbilityGas treatmentHeterogenous catalyst chemical elementsActive componentMercury oxidation
Disclosed are a catalyst for synergistic control of oxynitride and mercury and a method for preparing the same. The catalyst includes the following components by mass percentage: a carrier: TiO2 72%-98.6%, active components: V2O5 0.1%-5%, WO3 1%-10%, Cr2O3 0.1%-5% and Nb2O5 0.1%-5%, and a co-catalyst of 0.1%-3%. The present invention can be used for reducing the oxynitrides in a flue gas, meanwhile oxidizing zero-valent mercury into bivalent mercury and then controlling the reactions, has relatively high denitration performance and also has high mercury oxidation performance; compared with current commercial SCR catalysts, the mercury oxidation rate of the catalyst is improved to a great extent, which can adapt to the requirements for mercury removal in China's coal-fired power plants, the conversion rate of SO2 / SO3 is relatively low, and the catalyst has a better anti-poisoning ability, and is a new catalyst with a low cost and high performance.
Owner:ZHEJIANG UNIV
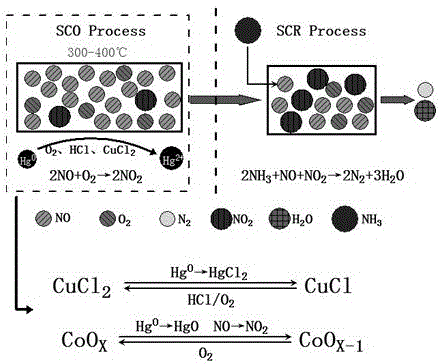
Composite catalyst based on denitration and demercuration reinforcing function of prepositive SCO smoke of coal-fired power plant and preparation method thereof
InactiveCN106311291AImprove performanceEasy to prepareGas treatmentPhysical/chemical process catalystsAdditive ingredientBULK ACTIVE INGREDIENT
The invention relates to a composite catalyst based on a denitration and demercuration reinforcing function of prepositive SCO smoke of a coal-fired power plant and a preparation method thereof. Main active ingredients of the composite catalyst are Co3O4 and CuCl2, and a carrier ingredient is Ce-doped TiO2. The preparation method includes: adopting a sol-gel method to prepare a Ce-doped TiO2 carrier, adopting an impregnation method to sequentially load Co3O4 and CuCl2. A selective catalytic oxidation unit using the catalyst as a main body is arranged at the front end of an SCR unit, oxidation of Hg0 does not be influenced by the ammonification process of the SCR unit, and high mercury oxidation removing efficiency can be realized in a chlorine-free condition. In addition, the catalyst can convert part of NO into NO2 while efficiently oxidizing Hg0, so that reaction efficiency of subsequent SCR process is improved remarkably. The catalyst is easy-to-get in raw material, simple in preparation method, easy-to-implement in technical process and conducive to industrial application.
Owner:WUHAN UNIV
Cerous phosphate based catalyst for zero-valent mercury oxidation, preparation method and applications thereof
ActiveCN104399499ARaw materials are readily availableEasy to operatePhysical/chemical process catalystsDispersed particle separationPhosphateCerium
The invention discloses a cerous phosphate based catalyst for zero-valent mercury oxidation. The catalyst is mainly composed of cerous phosphate, and comprises modification components; wherein the modification components are composed of at least one oxide of cobalt, manganese, copper, iron, vanadium, cerium, molybdenum, tin, and the like. The invention also discloses a preparation method and applications of the catalyst. The preparation process is simple, and the operation is convenient. Compared to the prior art, the catalyst has the following advantages: (1) the active components of the catalyst are all common metal oxides, the common metal phosphate is taken as the carrier, the raw materials are simple and easily available, and the operation is convenient; (2) the catalyst has a very good sulfur-resistant performance; (3) the CePO4 catalyst, which has been modified by metal oxides, is well adapt to the flue gas. The provided catalyst can fully utilizes the NO in flue gas to greatly promote the oxidation of zero-valent mercury. The great dependence on HCl of conventional catalysts is gotten rid of.
Owner:ZHEJIANG UNIV
Low temperature desulfurizing, dedusting, mercury-removal, oxidation absorption reduction denitration integrated tower and purification technology
InactiveCN105289258AMultiple conversionsAvoid enteringUsing liquid separation agentAir quality improvementWater bathsAtmospheric air
A low temperature desulfurizing, dedusting, mercury-removal, oxidation absorption reduction denitration integrated tower and its purification technology are disclosed. The tower is structurally characterized in that a first water tank and a third water tank are filled with an alkali absorption liquid; a second water tank is filled with a liquid oxidizing agent absorption liquid; the tower body is composed of four towers; a self-excitation device is arranged below a first tower and a second tower; the first tower is provided with a smoke inlet, an oxygen injection opening, a spray thrower, a packing chamber and an impact opening from top to bottom; the second tower is equipped with a packing chamber, a spray thrower, an oxygen injection opening and an oxidizing agent injection opening from bottom to top; a third tower is provided with a spray thrower and a packing chamber from top to bottom; and a fourth tower is provided with a packing chamber, a tower pore plate, a spray thrower and a smoke outlet from bottom to top. Smoke enters the tower through the smoke inlet and passes through the four-stage towers to undergo multi-stage purification by oxygenation conversion and spray water-bath. Harmful substances such as sulfur dioxide, dust, mercury and the like are purified while nitrogen oxide undergoes harmless treatment by technical means of oxidation, absorption and reduction. Therefore, harmful gas in smoke is thoroughly removed, and pollutants are prevented from entering the atmosphere.
Owner:阎君
Device for removing gas mercury by photo-catalysis and application thereof
The invention discloses a device for removing gas mercury by photo-catalysis, which adopts the method for loading nanometer TiO2-SiO2 on fibers of a titanium mesh and installing a UV ultraviolet source on the middle part. The gaseous mercury passing through the fibers of the titanium mesh is oxidized as Hg2+ through arranging multi-branched double-cylinder pipe which can be detached in sections in a gas duct, and then the gaseous mercury can be removed through the lime slurry of equipment for removing sulfur by wet process. The reaction sections of the device for removing the gas mercury by photo-catalysis in the invention are separately connected, so the reaction sections can be conveniently detached and changed, the removing efficiency is over 60%, and the economical efficiency is good. Simultaneously the complete device has the advantages of little taken space and good economical efficiency, and the device can be conveniently detached and changed. The device can be applied to the relative fields of removing the gas mercury from power plants, industrial boilers and coal combustion facilities, and has wide prospect on popularization and application of environmental mercury pollution treatment technology.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
Method and device for removing mercury from flue gas through optical radiation chlorine atoms and hydroxide radicals
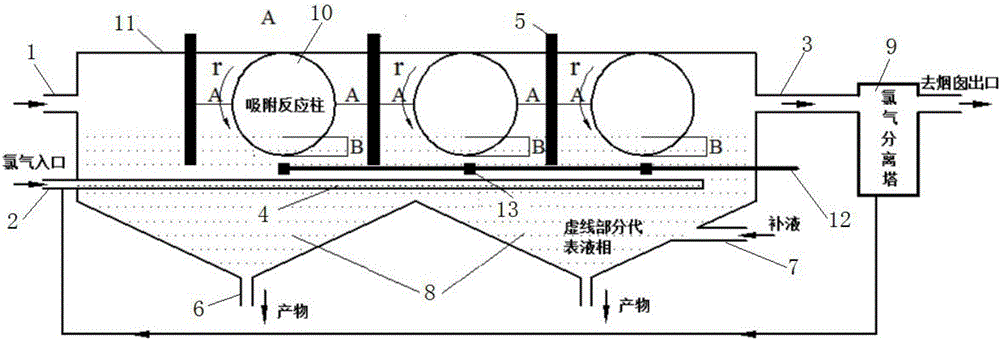
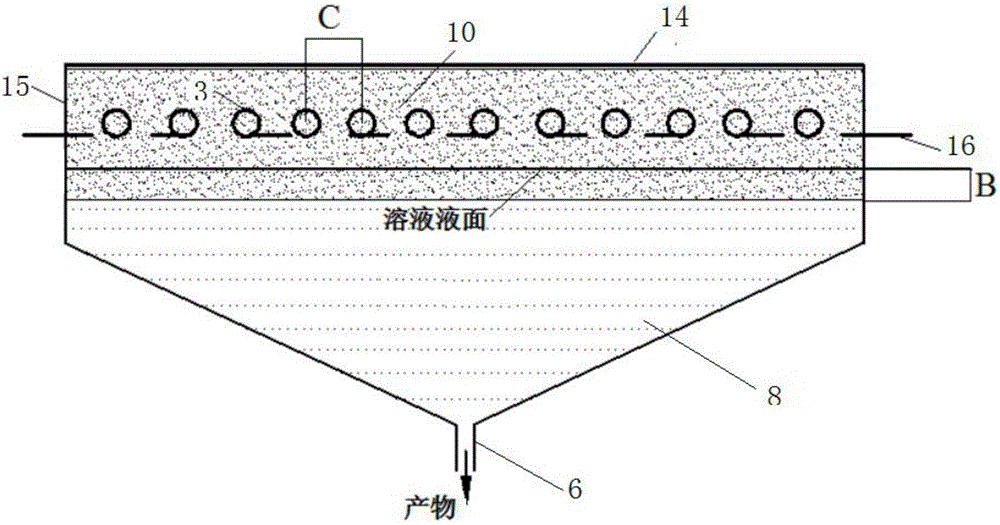
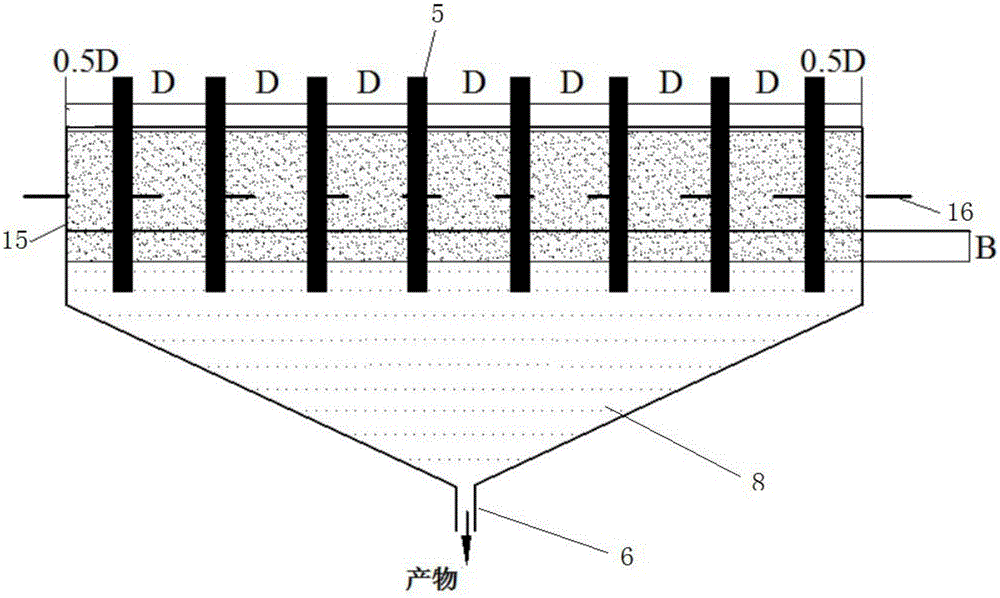
ActiveCN105833685ASimple equipmentReduce initial investmentGas treatmentDispersed particle separationEnvironmental chemistryFlue gas
The invention provides a method and device for removing mercury from flue gas through optical radiation chlorine atoms and hydroxide radicals. The mercury-containing flue gas from a boiler enters an optical radiation adsorption washing bed and is in contact with the active carbon fibers on an adsorption reaction column; chlorine forms a hypochlorous acid solution in water; the active carbon fibers on the reaction column are rotated and soaked into the solution and then absorb hypochlorous acid on the surface; under the radiation of ultraviolet light, the hypochlorous acid on the surfaces of the active carbon fibers is decomposed into high-activity chlorine atoms and hydroxide radicals; the mercury in flue gas has oxidizing reaction with the hydroxide radicals and is fixed on the surfaces of the active carbon fibers; and the reaction column is rotated and soaked into the solution again, the mercury oxidative products absorbed on the surfaces fall off and then enter the solution, so that the continuous demercuration and washing processes are completed. The mercury-removing oxidative products are precipitated and separated and then are recycled, and the chlorine remaining in the flue gas is absorbed, separated and then recycled. The system has ultrahigh oxidability, the mercury-removing rate reaches 100%, the removing process is free from secondary pollution, and thus the method has wide market application prospects.
Owner:JIANGSU UNIV
System for processing exhaust gas, and method for processing exhaust gas
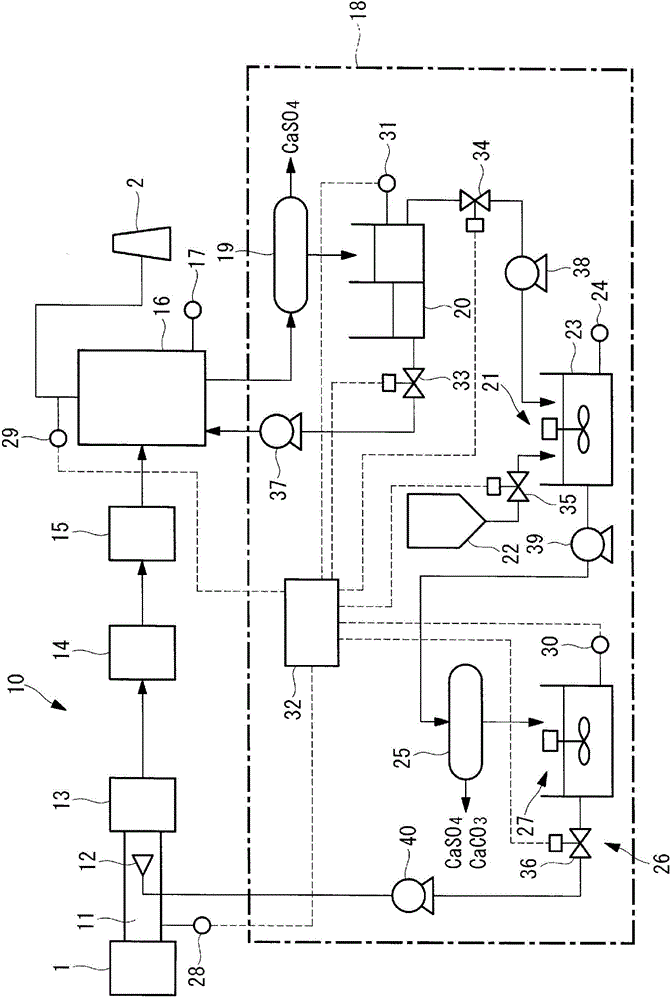
InactiveCN104619400ALow running costGas treatmentEmission preventionDiammonium carbonateMercury oxidation
A flue gas treatment system and a flue gas treatment method capable of drastically reducing operating cost and an amount of waste water and capable of controlling an amount of a NOx reducing agent and a mercury oxidation agent to be supplied are provided. The flue gas treatment system sprays an aqueous solution containing ammonium halide into a flue, reduces NOx and oxidizes mercury in a deNOx section, and removes SOx and the mercury in a desulfurization section. The flue gas treatment system adds at least one of ammonium sulfate and ammonium carbonate to waste water which is discharged from the desulfurization section and from which CaSO4 is separated to generate the ammonium halide. The waste water containing the ammonium halide is sprayed as the aqueous solution.
Owner:MITSUBISHI POWER LTD
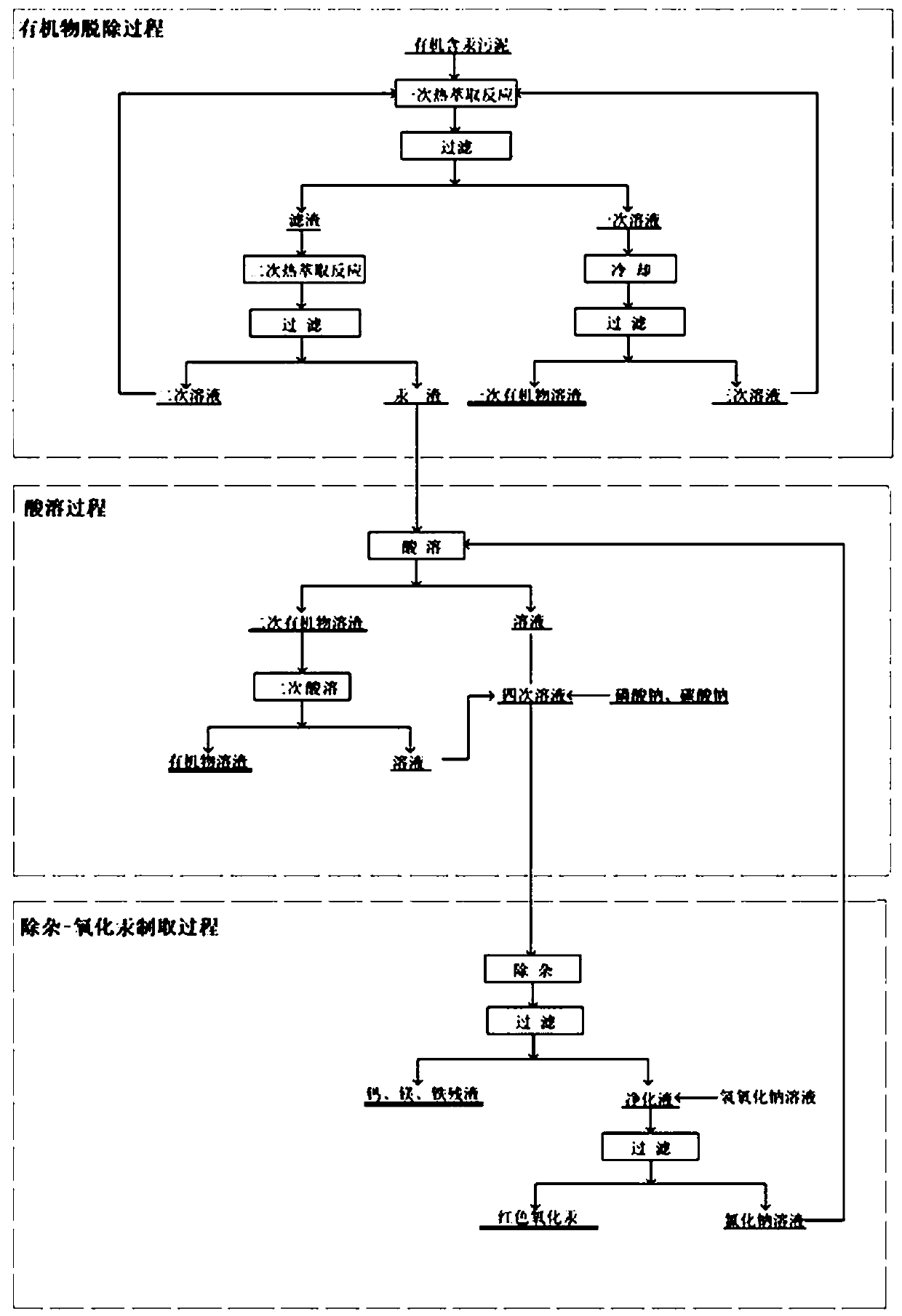
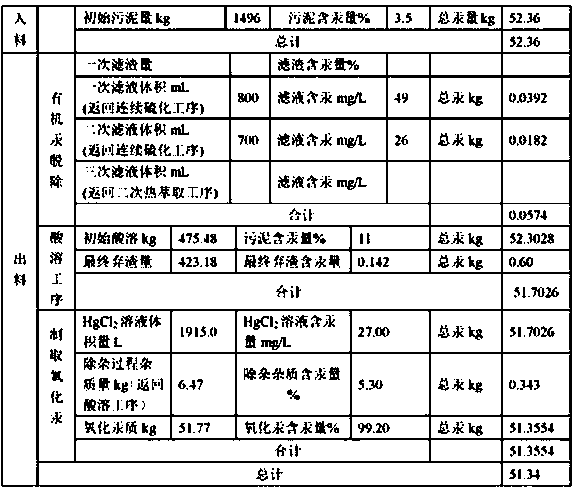
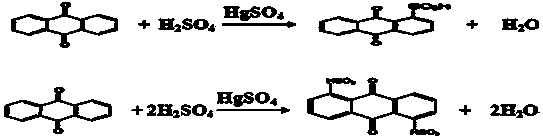
Method for directly preparing mercury oxide from mercury-containing organic sludge
ActiveCN111268718AEfficient removalHigh removal rateSludge treatmentWater contaminantsAnthraquinonesWarm water
The invention provides a method for directly preparing mercury oxide from mercury-containing organic sludge. The method comprises an organic matter removal process, an acid dissolution process and animpurity removal-mercury oxide preparation process. By utilizing the high difference of temperature gradient dissolution of anthraquinone byproduct organic matters and mercury compounds in water, warmwater is adopted for thermal extraction in organic matter removal, and the total removal rate is greater than 99%; and a HCl-NaClO3 mixed solution is adopted to dissolve valence mercury and oxidize elemental mercury so as to ensure that the mercury content of the residues is lower than 0.08%. Impurities in an acid solution containing HgCl2 are removed by adopting a Na2CO3-Na3PO4 combined technology; and the removal rates of calcium, magnesium and iron impurities are respectively greater than 95%, 93% and 96%. A full-wet mercury oxide preparation process is adopted, the prepared mercury oxideproduct is high in quality and can be directly returned to the production process, and the metal mercury purchase quantity of enterprises is reduced; and high-quality utilization of mercury-containingorganic sludge can be realized, and the secondary pollution problem of pyrogenic mercury extraction is thoroughly solved.
Owner:沈阳鑫迪环境技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com