Catalyst for mercury oxidation and preparation method and purpose thereof
A catalyst, mercury oxidation technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the problems of poor catalytic effect, low space velocity, and strong dependence on HCl And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
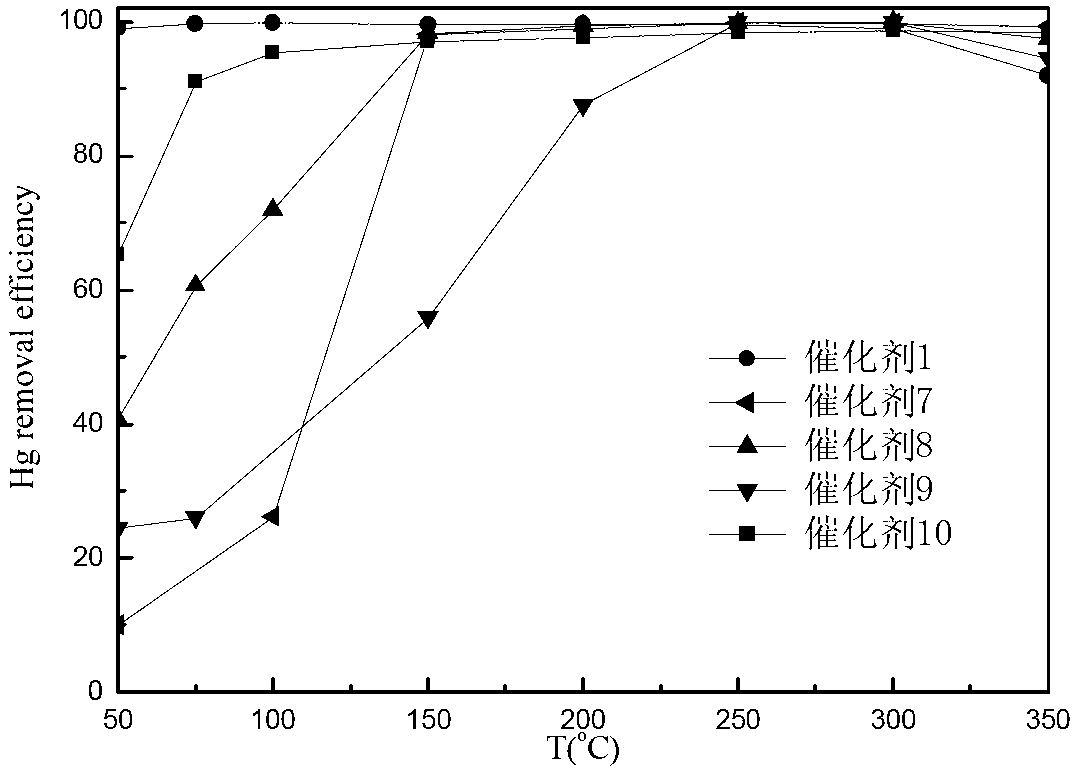
[0105] Take by weighing 0.23g copper nitrate trihydrate and dissolve to obtain an aqueous solution of copper, weigh 6.00g titanium dioxide (TiO 2 ) into it, stirring continuously for 5 hours, 70°C rotary evaporation, 110°C overnight drying, 450°C roasting for 5h, cooling, grinding, tableting, sieving, and 40-60 mesh catalysts were sieved and recorded as Catalyst 1. Change the quality of copper nitrate trihydrate to 0.11g, 0.68g, 1.13g, 1.59g, 2.27g, and the amount of titanium dioxide remains unchanged. Under the same circumstances, copper oxide catalysts with different loadings are obtained, and the active component is calculated by copper element 0.5wt%, 3wt%, 5wt%, 7wt%, 10wt%, respectively, recorded as catalyst 2, 3, 4, 5, 6.
[0106] Control experiment: Weigh 0.19g of cerium nitrate hexahydrate, 0.39g of manganese nitrate solution with a mass fraction of 50%, 0.30g of cobalt nitrate hexahydrate and 0.43g of ferric nitrate nonahydrate and dissolve them respectively to obtai...
Embodiment 2
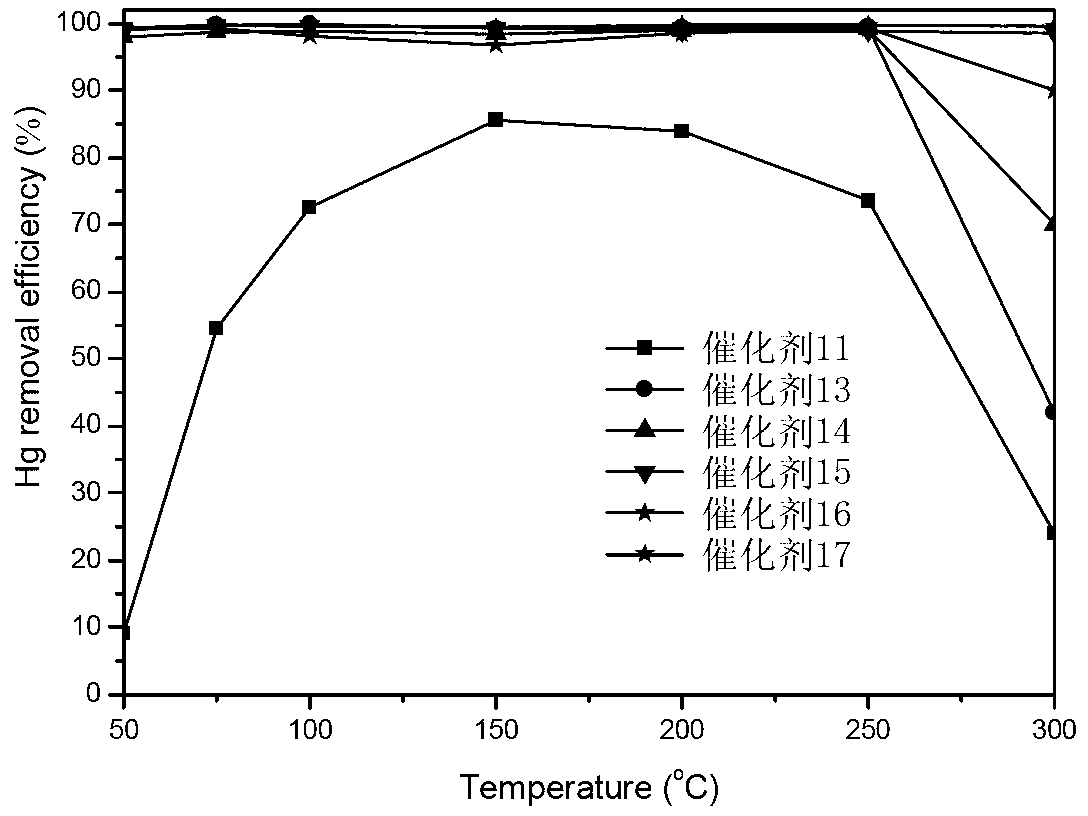
[0108] Take by weighing 0.16g copper chloride dihydrate and dissolve to obtain an aqueous solution of copper chloride, weigh 6.00g of titanium dioxide (TiO 2 ) into it, continuously stirred for 5h, 70°C rotary evaporation, 110°C overnight drying, 400°C roasting for 5h, after cooling, grinding, tableting, sieving, and 40-60 mesh catalyst was sieved to prepare the active component ( Calculation of copper element) is a copper chloride catalyst of 1wt%, denoted as catalyst 11. Change the quality of copper chloride dihydrate to 0.08g, 0.48g, 0.79g, 1.12g, 1.59g, and the amount of titanium dioxide remains unchanged. Under the same circumstances, copper chloride catalysts with different loadings are obtained, and the active substance is calculated by copper element The components are 0.5wt%, 3wt%, 5wt%, 7wt%, and 10wt%, respectively, and are recorded as catalysts 12, 13, 14, 15, and 16, respectively.
Embodiment 3
[0110] Take by weighing 0.48g copper chloride dihydrate and dissolve with 0.21g potassium chloride to obtain a mixed aqueous solution of copper chloride and potassium chloride, weigh 6.00g titanium dioxide (TiO 2 ) into it, continuously stirred for 5h, 70°C rotary evaporation, 110°C overnight drying, 400°C roasting for 5h, after cooling, grinding, tableting, and sieving, the active component (calculated as copper element) was 3wt% while Cu : CuCl with K (molar ratio) 1:1 2 -KCl / TiO 2 , and sieve the 40-60 mesh catalyst for future use, and record it as catalyst 17. Also weigh 0.48g copper chloride dihydrate, change the quality of potassium chloride to 0.01g, 0.11, 0.42, and 0.84g respectively, and respectively prepare the active component (calculated by copper element) as 3wt% and Cu:K (mol ratio) respectively 1:0.1, 1:0.5, 1:2, 1:4 CuCl 2 -KCl / TiO 2 The catalysts are denoted as catalysts 18, 19, 20, and 21, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| oxidation efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com