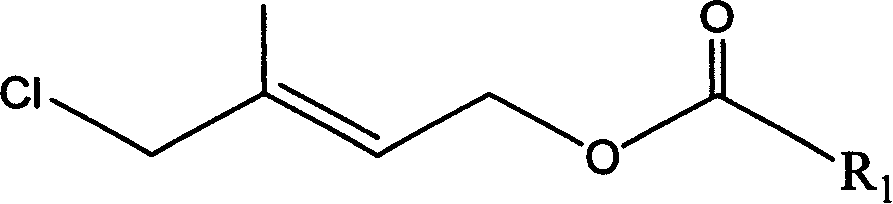
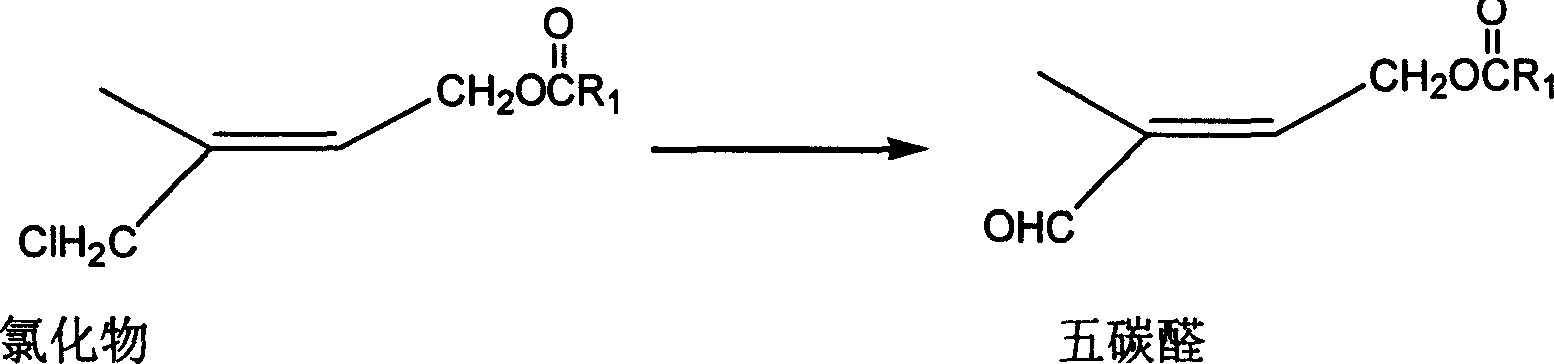
Method for preparing 1-chloro-2-methyl-4-alkylacyloxy-2-butene
一种烃酰氧基、甲基的技术,应用在药物化学领域,能够解决产物收率和含量差、杂质增多、产品含量差等问题,达到反应体系温和、含量和收率高、杂离子少的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of 1-chloro-2-hydroxyl-2-methyl-3-butene and 1-chloro-2-methyl-4-hydroxyl-2-butene mixture
[0040] Put a 500ml three-necked bottle equipped with a thermometer and a solid feeding port into an alcohol cooling bath; add 68g (1mol) of isoprene, 100ml of water and 0.5g of inhibitor hydroquinone; Control the temperature at the feeding port and add trichloroisocyanuric acid (90% available chlorine) in batches, a total of 58g (0.25mol), and add it in about 1 hour, then continue to keep warm and stir for 1 hour, filter, wash the filter cake with 15ml of water, and it is a loose white powder Solid, weighing 31 g (0.24 mol) after drying. The filtrates were combined and allowed to stand for separation. The organic layer was lower than 40°C to recover unreacted isoprene under reduced pressure to obtain 90 g of crude product. Gas phase analysis showed that the total content of the product was 92.5%, and the yield was 92.1%. It can be directly used in the nex...
Embodiment 2
[0041] Example 2: Preparation of 1-chloro-2-methyl-4-acetoxy 2-butene by esterification rearrangement reaction
[0042] In a 250ml three-necked flask, add 63g of the crude product (content 92.5%, 0.48mol) and 80g (0.78mol) of acetic anhydride, stir and add 1g of p-toluenesulfonic acid, heat up to 60°C and stir for 5 hours, cool to room temperature, add 100ml water layer, discard the upper waste water, the lower organic layer is washed with 100ml water and then stratified to obtain 63g (content 90%) of crude product chloride, after rectification (80-90°C / 1mmHg), 55g colorless transparent liquid (content 93.5%), yield 66%. GC-MS (m / e): 127, 102, 84, 67, 43 (100%), 29; IR (ν / cm -1 ): 1735 (-OCO-, carbonyl); 1230 (-C-O-CO-, ν as ), 1035 (-C-O-CO-, ν s ); 1 HNMR (500MHz, CDCl 3 )δ (ppm): 1.83 (s, 3H, -CH 3 ); 2.06(s,3H,-COCH 3 ); 4.01 (2H, Cl-CH 2 -); 4.62 (2H, =CH 2 ); 5.69 (1H, -CH=); DEPT: δ (ppm): 124.019 (1H, =CH-); 62.535 (2H, -OCH 2 -); 50.135 (2H, -CH 2 -Cl); 21....
Embodiment 3
[0043] Embodiment 3: material proportioning, operating temperature and aftertreatment are the same as embodiment 1, the difference is that the water for reaction is the waste water layer in embodiment 1. 93g of crude product was obtained, gas phase analysis showed that the total product content was 89.5%, and the yield was 92.1%. The filter cake is a loose white powder solid, weighing 30.5 grams (0.236 mol) after drying, and the water layer is 98 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com