A method for preparing 2-chloro-3-cyano-4-picoline
A picoline and cyano technology, applied in the field of preparing 2-chloro-3-cyano-4-picoline, can solve the problems of phosphorus oxychloride easy to corrode production equipment, not suitable for industrial production, and environmental protection , to achieve the effect of shortening processing time, low cost and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
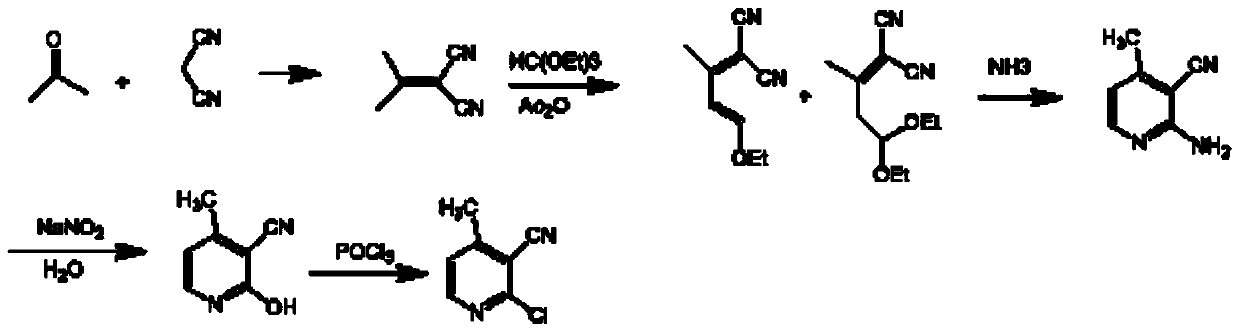
[0039] Example 1: 4,4-dicyano-3-methyl-3-butenal dimethyl acetal and 1,1-dicyano-4-methoxy-2-methyl-1,3- Preparation of mixtures of butadiene
[0040]
[0041] Add 100.0g of sodium methoxide and 1500mL of toluene to the reaction flask, add 140mL of acetone dropwise at room temperature, and add 140mL of methyl formate dropwise. After the dropwise addition, keep stirring for 30 minutes; heat up to 50°C and continue stirring for 3 hours; naturally cool down to At room temperature, a light yellow suspension was obtained.
[0042] In another reaction bottle, add 800mL of 15wt% hydrogen chloride methanol solution, slowly add the light yellow suspension obtained in the previous step reaction, after the dropwise addition, naturally warm up to room temperature, and continue to stir for 4 hours; cool down to 0°C, drop Add 28wt% sodium methoxide methanol solution; filter, and concentrate the filtrate to 1 / 3 of the original volume under reduced pressure to obtain a mixture of acetoace...
Embodiment 2
[0044] Embodiment 2: Preparation of 2-chloro-3-cyano-4-methylpyridine
[0045]
[0046] Under ice-bath conditions, feed hydrogen chloride gas into 300 mL of ethanol until saturation, slowly add the reddish-brown oily substance obtained in Example 1, after the addition, continue the heat preservation reaction at 10 ° C for 8 hours; add the reaction mixture dropwise to 1.5 In L water, keep warm and continue stirring for 1 hour; filter with suction, wash the filter cake with ice water, and dry in vacuo to obtain 178.0 g of 2-chloro-3-cyano-4-methylpyridine with an HPLC purity of 99.5%.
Embodiment 3
[0047] Embodiment 3: Preparation of 2-chloro-3-cyano-4-methylpyridine
[0048] Under ice-bath conditions, first add 480 mL of ethanol solution to the reaction flask, then slowly add the reddish-brown oil obtained in Example 1, after the addition is complete, add 300 grams of acetyl chloride dropwise at 10°C to generate hydrogen chloride, and continue the insulation reaction for 50 hour; the reaction mixture was added dropwise to 2L of water, and the insulation continued to stir for 1 hour; suction filtration, the filter cake was washed with ice water, and dried in vacuo to obtain 174.5 g of 2-chloro-3-cyano-4-picoline, HPLC purity 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com