A kind of cerium oxide/iron oxide/mesoporous silicon nanocomposite material and its preparation method and application
A nano-composite material, iron oxide nano technology, applied in the direction of drug combination, pharmaceutical formula, preparation for in vivo test, etc., can solve the problem of no effective treatment method, no clinical diagnosis specific sign, etc. Good potential for clinical translation, good biocompatibility, and controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthesis and ligand conversion of cerium oxide nanocrystals: 0.4 g of cerium acetate hydrate and 3.2 g of oleylamine were added to 15 ml of xylene, stirred at room temperature for 4 hours, and raised to 90 degrees Celsius at a heating rate of 2 degrees Celsius per minute ; Inject 1ml of deionized water into the reaction system protected by an inert gas, age for three hours, precipitate with acetone, and centrifuge to obtain cerium oxide nanocrystals.
[0042] 15 mg of the synthesized cerium oxide nanocrystals, 0.5 g of 2-bromoisobutyric acid and 0.05 g of citric acid were sequentially added to a mixed solvent of 7.5 ml of chloroform and 7.5 ml of N,N-dimethylformamide and stirred for 24 hours. have to.
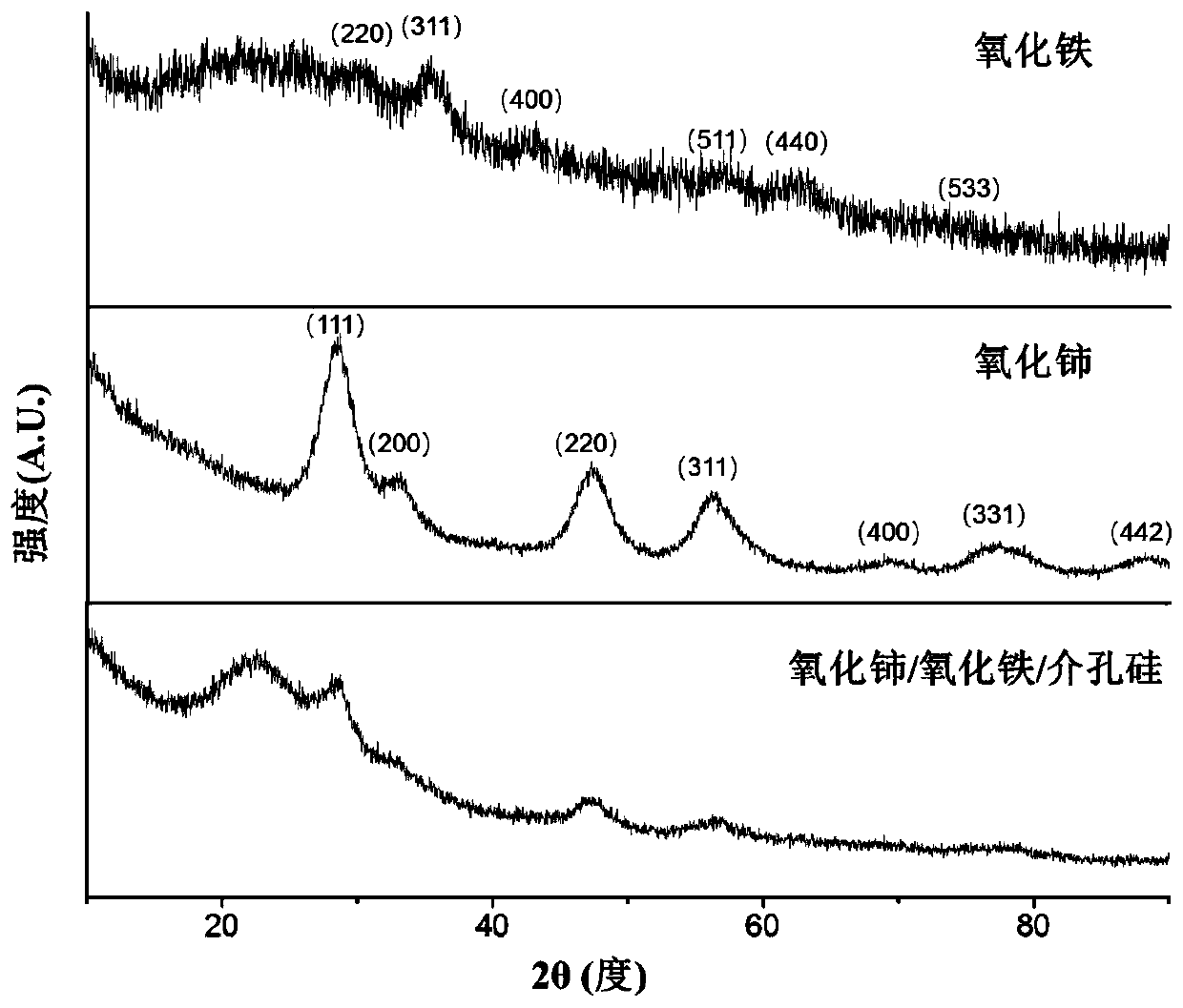
[0043] X-ray diffraction was performed on the obtained ligand-converted ceria nanocrystals, as shown in the attached figure 1 shown; and the morphology of ligand-converted cerium oxide nanocrystals was characterized by transmission electron microscopy, as shown in...
Embodiment 2
[0052] (1) Synthesis and ligand conversion of cerium oxide nanocrystals: 0.4 g of cerium acetate hydrate and 3.6 g of oleylamine were added to 15 ml of xylene, stirred at room temperature for 6 hours, and raised to 95 degrees Celsius at a heating rate of 2 degrees Celsius per minute ; Inject 1ml of deionized water into the reaction system protected by an inert gas, age for three hours, precipitate with acetone, and centrifuge to obtain cerium oxide nanocrystals.
[0053] 10 mg of the synthesized cerium oxide nanocrystals, 0.5 g of 2-bromoisobutyric acid and 0.05 g of citric acid were sequentially added to a mixed solvent of 7.5 ml of chloroform and 7.5 ml of N,N-dimethylformamide and stirred for 12 hours. have to.
[0054] (2) Synthesis and ligand conversion of iron oxide nanocrystals: 2 g of iron oleate, 1 g of oleic acid, and 2 g of oleyl alcohol were added to 10 g of octadecene. Stir at room temperature, and rise to 300 degrees Celsius at a heating rate of 10 degrees Celsi...
Embodiment 3
[0059] (1) Synthesis and ligand conversion of cerium oxide nanocrystals: 0.4 g of cerium acetate hydrate and 2.8 g of oleylamine were added to 15 ml of xylene, stirred at room temperature for 3 hours, and raised to 85 degrees Celsius at a heating rate of 2 degrees Celsius per minute ; Inject 1ml of deionized water into the reaction system protected by an inert gas, age for three hours, precipitate with acetone, and centrifuge to obtain cerium oxide nanocrystals.
[0060] 20 mg of the synthesized cerium oxide nanocrystals, 0.5 g of 2-bromoisobutyric acid and 0.05 g of citric acid were sequentially added to a mixed solvent of 7.5 ml of chloroform and 7.5 ml of N,N-dimethylformamide and stirred for 4 hours. have to.
[0061] (2) Synthesis and ligand conversion of iron oxide nanocrystals: 2g iron oleate, 1.2g oleic acid, and 2.5g oleyl alcohol were added to 10g octadecene. Stir at room temperature, and rise to 250 degrees Celsius at a heating rate of 10 degrees Celsius per minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com