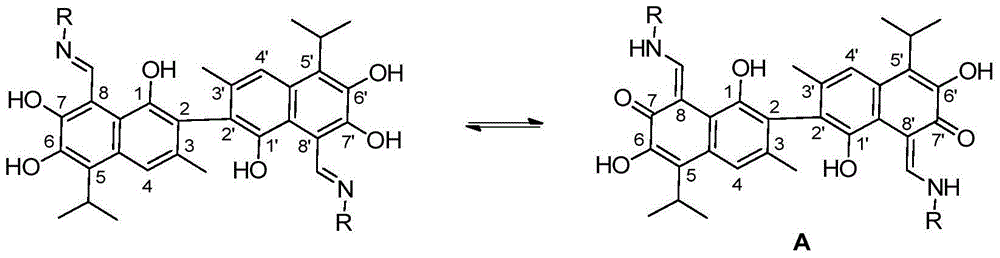
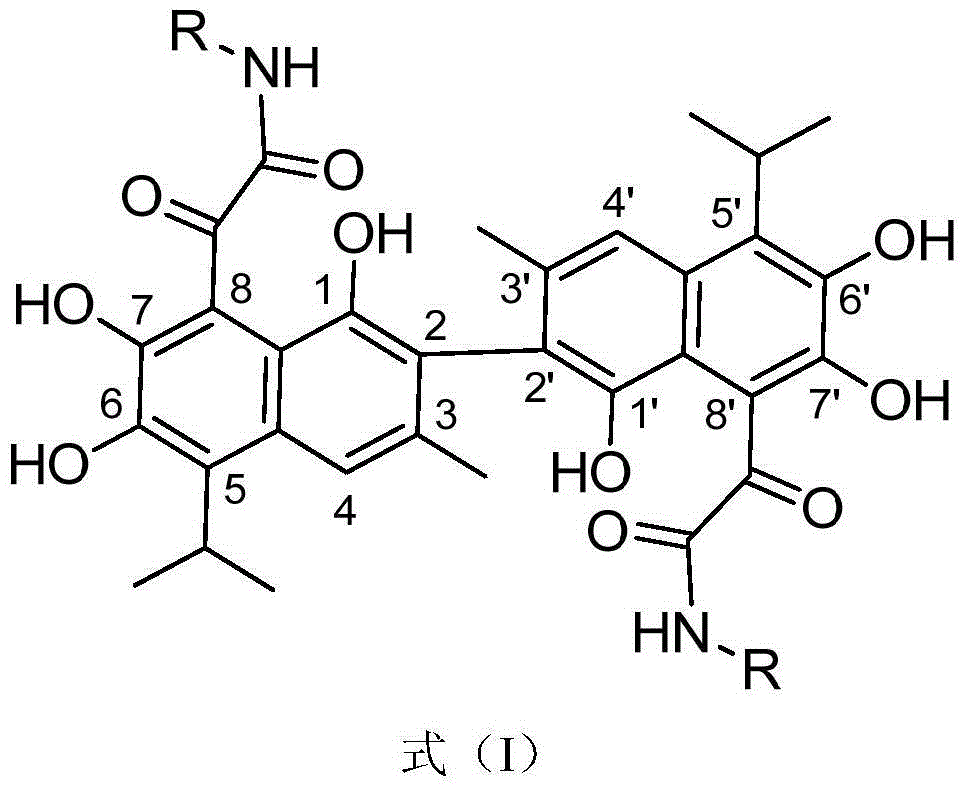
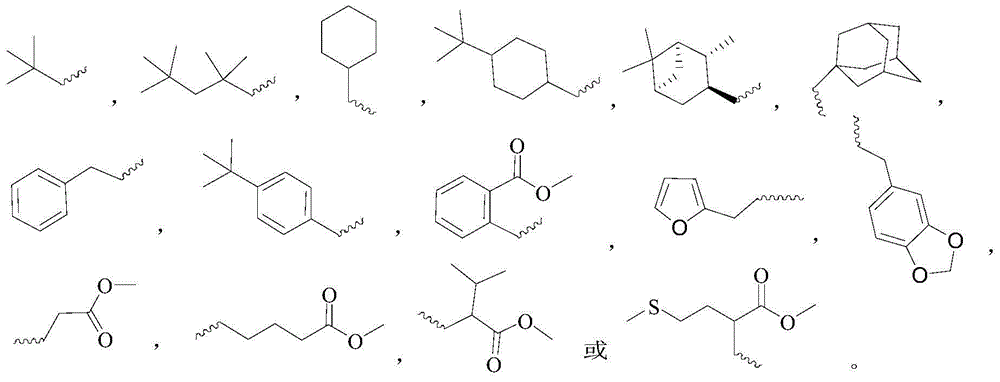
Novel gossypol derivatives, and preparation method and antineoplastic application thereof
A derivative, the technology of gossypol, applied in the field of novel gossypol derivatives and their preparation, anti-tumor applications, can solve the problems such as the inability to exert the maximum biological activity of the compound, the reduction of antiviral biological activity, the influence of antiviral activity, etc., and achieve excellent Biological activity, resolving toxic aldehyde groups, simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: 2,2'-(1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2, Preparation of 2'-binaphthyl]-8,8'-diyl)bis(N-benzyl-2-oxoacetamide)
[0031] Take (346mg, 0.6mmol, 1.0eq) gossypol acetate and place it in a 10mL single-necked round-bottomed flask, add 2.5mL of ethanol and 100uL of water, and add (2.2eq) of isocyanide 1( ), the addition was completed, the reflux condenser was installed, and the temperature was slowly raised to 80°C. After 24 hours, the TCL detection reaction was basically complete. After standing still, the solid was precipitated, filtered with suction, and the filter residue was washed 3 times with ethanol to obtain a yellow solid with a yield of 53%.
[0032]
[0033] 1 H NMR (400MHz, CDCl 3 )δ=13.60(s,1H),9.73(d,J=12.2,1H),7.95(s,1H),7.59(s,1H),7.38–7.27(m,5H),5.59(s,1H) ,4.67(d,J=4.3,2H),3.81–3.65(m,1H),2.11(s,3H),1.52(dd,J=6.8,4.8,6H). 13 C NMR (100MHz, CDCl 3 )δ=172.98,163.00,149.04,147.12,136.24,132.01,129.07,128.24,127.60,...
Embodiment 2
[0034] Example 2: 2,2'-(1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2, Preparation of 2'-binaphthyl]-8,8'-diyl)bis(N-cyclohexyl-2-oxoacetamide)
[0035] Take (346mg, 0.6mmol, 1.0eq) gossypol acetate and place it in a 10mL single-necked round-bottomed flask, add 2.5mL of ethanol and 100uL of water, and add (2.2eq) of isonitrile 2( ), the addition was completed, the reflux condenser was installed, and the temperature was slowly raised to 80°C. After 24 hours, the TCL detection reaction was basically complete. After standing still, the solid was precipitated, filtered with suction, and the filter residue was washed 3 times with ethanol to obtain a yellow solid with a yield of 66%.
[0036]
[0037] 1 H NMR (400MHz, CDCl 3 )δ=13.40(s,1H),9.72(d,J=7.7,1H),8.04(s,1H),7.59(s,1H),5.64(s,1H),3.86–3.64(m,1H) ,3.48–3.28(m,1H),2.12(s,3H),2.00(m,2H),1.85–1.75(m,2H),1.66–1.57(m,2H),1.54–1.48(m,7H) ,1.39–1.23(m,3H). 13 C NMR (100MHz, CDCl 3)δ=172.12, 160.88, 148.90,...
Embodiment 3
[0038] Example 3: 2,2'-(1,1',6,6',7,7'-hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2, Preparation of 2'-binaphthyl]-8,8'-diyl)bis(N-(1-adamantyl)-2-oxoacetamide)
[0039] Take (346mg, 0.6mmol, 1.0eq) gossypol acetate and place it in a 10mL single-necked round-bottomed flask, add 2.5mL of ethanol and 100uL of water, and add (2.2eq) of isonitrile 3( ), the addition was completed, the reflux condenser was installed, and the temperature was slowly raised to 80°C. After 24 hours, the TCL detection reaction was basically complete. After standing still, the solid was precipitated, filtered with suction, and the filter residue was washed 3 times with ethanol to obtain a gray-yellow solid with a yield of 70%.
[0040]
[0041] 1 H NMR (400MHz, CDCl 3 )δ=13.57(d,J=15.0,1H),9.80(d,J=12.5,1H),7.61(s,1H),5.60(brs,1H),3.79–3.66(m,1H),2.17( m,3H),2.12(s,3H),1.94(m,5H),1.82–1.62(m,7H),1.57–1.49(m,6H). 13 C NMR (100MHz, CDCl 3 )δ=171.77, 158.07, 148.84, 147.30, 131.49, 128.87, 126.94...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com