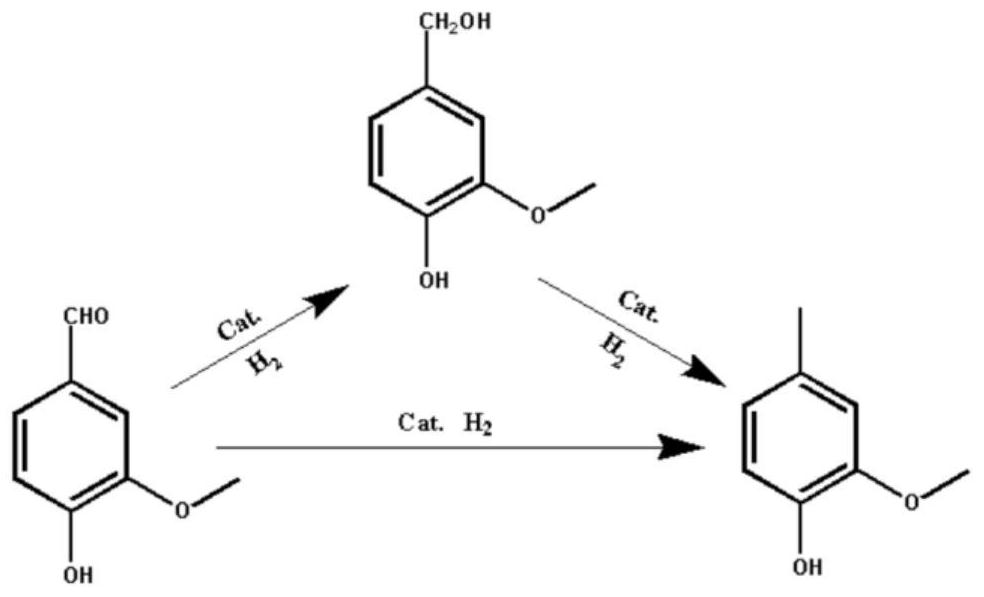
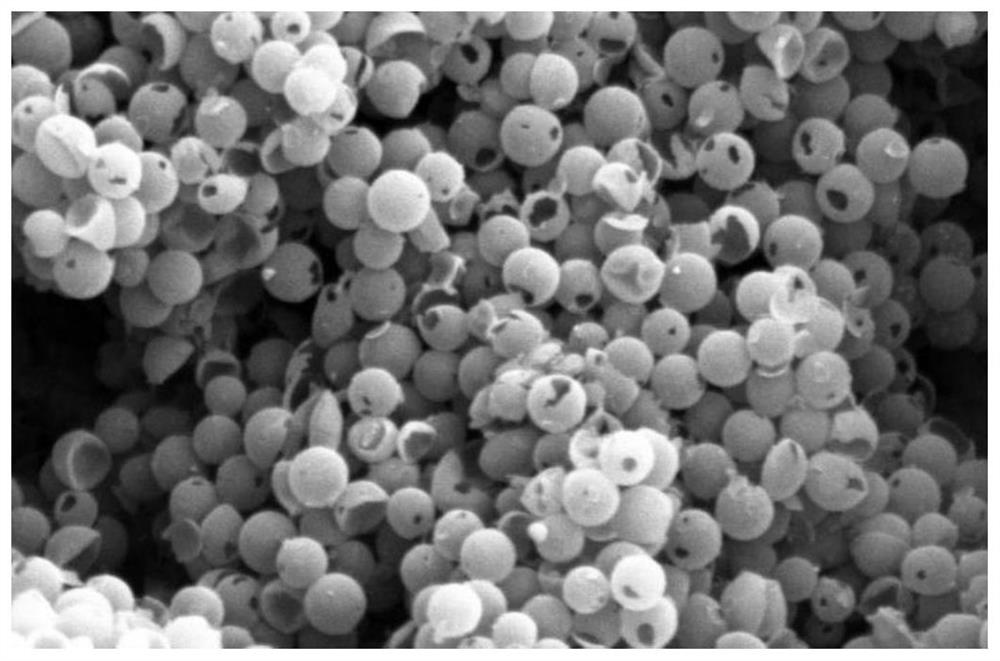
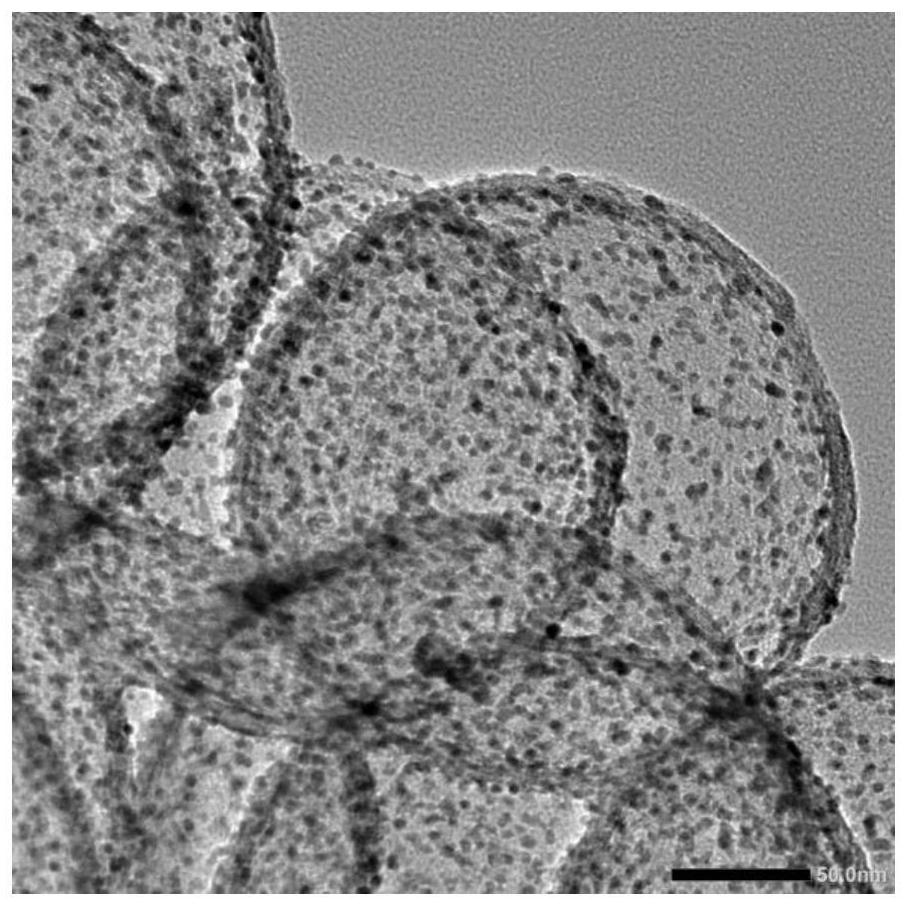
Preparation method of 2-methoxy-4-methylphenol based on selective hydrodeoxygenation of vanillin
A technology of selective hydrogenation and methyl phenol, applied in the preparation of organic compounds, chemical instruments and methods, catalyst activation/preparation, etc., can solve the problems of low energy efficiency, high temperature and high pressure, high hydrogen pressure, etc., and achieve simple reaction process, The effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~ Embodiment 9
[0036] Precipitation method: Dopamine hydrochloride and silica (2:1) were polymerized under a magnetic stirrer for 20 h, washed and dried, and calcined to 1000° C. under a nitrogen atmosphere. Remove the silica with NaOH solution, wash and dry by centrifugation, weigh 0.05g of the dried sample, add 20ml of deionized water, and adjust the pH of the solution to 9-10. The mass content of the catalyzed addition is 5%. After ultrasonic dispersion, sodium borohydride is added, and the molar ratio of sodium borohydride and the metal source is 30: 1. In the presence of sodium borohydride, it is fully reduced for 5h, washed and dried to obtain the obtained solution. described catalyst.
[0037] 0.01g, 0.005g, 0.008g or 0.015g of the above-mentioned deposition-precipitation method-Pt@CNS catalyst (wherein Pt: 5wt%, calcination temperature is 1000 ℃), 0.076g vanillin (vanillin and catalyst The mass ratios were 5:1, 7.6:1, 15.2:1, and 9.5:1) and 5 mL of isopropanol were added to a 50 mL ...
Embodiment 10
[0049] Dopamine hydrochloride and silica (mass ratio: 2:1 were polymerized under a magnetic stirrer for 20h, washed and dried, and calcined to a target temperature of 900°C under a nitrogen atmosphere). Use a certain concentration of NaOH solution to remove silica, wash and dry, weigh 0.05g of the dried sample, add 20ml of deionized water, and add chloroplatinic acid. The amount of nitric acid target added in the catalyst is the mass of the active component in the catalyst The content is 4.25%. After ultrasonic dispersion, sodium borohydride was added, the molar ratio of sodium borohydride and metal source was 30:1, fully reduced in the presence of sodium borohydride for 6 hours, washed and dried to obtain the catalyst.
[0050] 0.01 g impregnation method-Pt@CNS catalyst (wherein Pt: 4.25 wt%, calcination temperature is 900 °C), 0.076 g vanillin (the mass ratio of vanillin and catalyst is 7.6:1) and 5 mL isopropanol were added to 50 mL glass In the test tube, after the gas wa...
Embodiment 11
[0053] Dopamine hydrochloride and silica (mass ratio of 1:1 were polymerized under a magnetic stirrer for 24 hours, washed and dried, and calcined to a target temperature of 900°C under a nitrogen atmosphere). Use a certain concentration of NaOH solution to remove silica, wash and dry, weigh 0.05g of the dried sample, add 20ml of deionized water, and add chloroplatinic acid. The amount of chloroplatinic acid added in the catalyst is the active component in the catalyst. The mass content is 2.5%. After ultrasonic dispersion, sodium borohydride was added, the molar ratio of sodium borohydride and metal source was 30:1, fully reduced in the presence of sodium borohydride for 6 hours, washed and dried to obtain the catalyst.
[0054] 0.01 g of impregnation method-Pt@CNS catalyst (wherein Pt: 2.5 wt%, calcination temperature is 900 ° C), 0.076 g of vanillin and 5 mL of isopropanol were added to the lining of a 30 mL autoclave, and high-purity hydrogen was introduced to replace 5 A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com