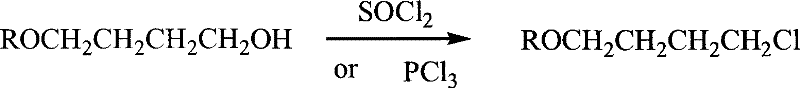Method for preparing 4-alkoxy-1-chlorobutane
A technology of alkoxy and chlorobutane, which is applied in the field of preparation of 4-alkoxy-1-chlorobutane, can solve the problems of difficult product refining, difficult catalyst, difficult cleaning, etc. The effect of easy handling and less usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1: the preparation of 4-methoxy-1-chlorobutane
[0015] Add 104g (1mol) 4-methoxy-butanol, 5 grams of tetramethylammonium chloride and 200ml hexanaphthene in the 500ml four-necked bottle that is furnished with thermometer and dropping funnel; Maintain reaction temperature 65~70 ℃, Stir and slowly add thionyl chloride 131g (1.1mol) dropwise for about 3 hours. After the waste acid tail gas is discharged, the alkali water is absorbed. After the drop is completed, keep stirring and react for 2 hours. Cool down to room temperature, wash the organic phase with 100ml of water, 50ml of saturated sodium bicarbonate, and 50ml of water, recover the solvent cyclohexane under reduced pressure, and distill and collect the fraction of bp114~117℃ to obtain the product 4-methoxy-1-chlorobutane 116.3 grams, gas chromatography content 99.1%, yield 94.1%.
[0016] 1 HNMRδ (ppm): 1.61 (m, 2H, -CH 2 -C-C-Cl), 1.75 (m, 2H, -CH 2 -C-Cl), 3.25(s, 3H, CH 3 -O), 3.35(t, 2H, O-CH ...
Embodiment 2
[0018] Embodiment 2: the preparation of 4-ethoxy-1-chlorobutane
[0019] Add 118g (1mol) of 4-ethoxyl-1-butanol, 8 grams of tetraethylammonium chloride and 200ml of cyclohexane in a 500ml four-necked bottle equipped with a thermometer and a dropping funnel; maintain the reaction temperature at 65-70 ℃, stirred and slowly added thionyl chloride 131g (1.1mol) dropwise, and the drop was completed in about 3 hours. After the waste acid tail gas was discharged, the alkaline water was absorbed, and after the drop was completed, it was incubated and stirred for 2 hours. Cool down to room temperature, wash the organic phase with 100ml of water, 50ml of saturated sodium bicarbonate, and 50ml of water, recover the solvent cyclohexane under reduced pressure, and distill and collect the bp83~86℃ / 100mmHg fraction to obtain the product 4-ethoxy-1-chloro 129.2 grams of butane, gas chromatography content 99.2%, yield 93.9%.
[0020] 1 HNMRδ (ppm): 1.09 (tri, 3H, CH 3 -), 1.63 (m, 2H, -CH ...
Embodiment 3
[0022] Embodiment 3: the preparation of 4-propoxy-1-chlorobutane
[0023] Add 132g (1mol) 4-propoxy-1-butanol, 8 grams of tetrabutylammonium chloride and 250ml toluene in the 500ml four-necked bottle that is furnished with thermometer and dropping funnel; Maintain reaction temperature 65~70 ℃, Stir and slowly add thionyl chloride 131g (1.1mol) dropwise for about 3 hours. After the waste acid tail gas is discharged, the alkali water is absorbed. After the drop is completed, keep stirring and react for 2 hours. Cool down to room temperature, wash the organic phase with 100ml of water, 50ml of saturated sodium bicarbonate, and 50ml of water, recover the solvent toluene under reduced pressure, and distill and collect the bp93~95℃ / 90mmHg fraction to obtain the product 4-propoxy-1-chlorobutane 139.5 grams, gas chromatography content 99.1%, yield 91.9%.
[0024] 1 HNMRδ (ppm): 0.84 (tri, 3H, CH 3 -), 1.53 (m, 2H, 2H, CH 3 -CH 2 * -CH 2 -O-), 1.59 (m, 2H, -CH 2 -C-C-Cl), 1.73 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com