Method for preparing decitabine
A technology of decitabine and citabine, which is applied in the field of compound preparation, can solve the problems of low preparation process yield, high production cost, unsuitability for large-scale industrial production, etc., and achieve easy availability of raw materials and high total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
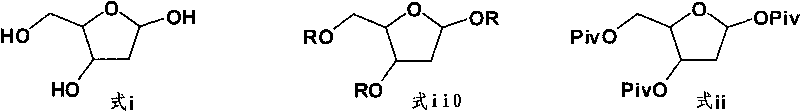
[0027] Example 1 Preparation of 1,3,5-tri-O-pivaloyl-2-deoxy-D-ribose (formula ii)
[0028] Add 20 g (149.11 mmol) of 2-deoxy-D-ribose in 100 mL of anhydrous pyridine to a 250 mL three-neck flask, slowly add 104.97 mL (517.41 mmol) of pivalic anhydride under nitrogen protection at 0-5°C, and rise to React at room temperature for 2 to 3 hours. The reaction of the raw material 2-deoxy-D-ribose was detected by TLC (dichloromethane:methanol=8:1 V / V). The solvent was evaporated under reduced pressure, the residue was dissolved in 400mL of dichloromethane, washed twice with saturated sodium bicarbonate solution (200mL×2), washed twice with saturated brine (200mL×2), dried over anhydrous sodium sulfate, filtered, evaporated After drying, 57.06 g (yield: 99.01%) of 1,3,5-tri-O-pivaloyl-2-deoxy-D-ribose (formula ii) was obtained, which was directly used in the next reaction.
Embodiment 2
[0029] Example 2 Preparation of 2-trimethylsilylamino-4-trimethylsilyloxy-1,3,5-triazine (formula iv)
[0030] Add 19.94g (177.89mmol) of 5-azacytosine, 90.14mL (427.27mmol) of freshly distilled hexamethyldisilazane and 99.70mL of anhydrous pyridine into a 500mL three-necked flask, and stir at room temperature for 10-15min. Add a catalytic amount of trimethylchlorosilane 1.12mL (8.89mmol) at room temperature, gradually raise the temperature to reflux at 110-120°C, keep the reaction for 3-4 hours, then cool down to room temperature naturally, evaporate the solvent under reduced pressure, add anhydrous Chloromethane 200mL, atmospheric distillation to take away the residual solvent. 44.71 g (yield: 98.01%) of 2-trimethylsilylamino-4-trimethylsilyloxy-1,3,5-triazine was obtained, which was directly used in the next reaction.
Embodiment 3
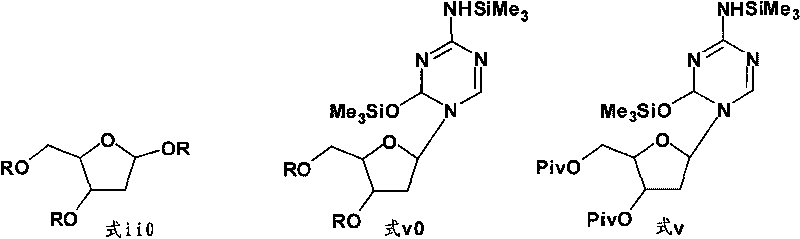
[0031] Example 3 Preparation of 1-(3,5-di-O-pivaloyl-2-deoxy-D-ribose)-5-azacytosine (formula v)
[0032] 57.06 g (147.64 mmol) of 1,3,5-tri-O-pivaloyl-2-deoxy-D-ribose (formula ii) as obtained in Example 1 and 2-trimethylsilyl as obtained in Example 3 Amino-4-trimethylsilyloxy-1,3,5-triazine (Formula iv) 40.51g (157.98mmol) was dissolved in 360mL of dichloromethane, stirred at room temperature for 10-15min, then cooled to 0-5°C , add TMSOTf5.34mL (29.53mmol) dropwise within 5~10min, while controlling the reaction temperature at 15~20°C. The reaction of tri-O-pivaloyl-2-deoxy-D-ribose was complete (petroleum ether: ethyl acetate=3:1 v / v). The reaction solution was washed twice with saturated sodium bicarbonate solution (200mL×2), the resulting aqueous phase was extracted three times with dichloromethane (100mL×3), the combined extracts were washed twice with saturated brine (200mL×2), and depressurized Evaporated to dryness, obtained 1-(3,5-di-O-pivaloyl-2-deoxy-D-ribose)-5-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com