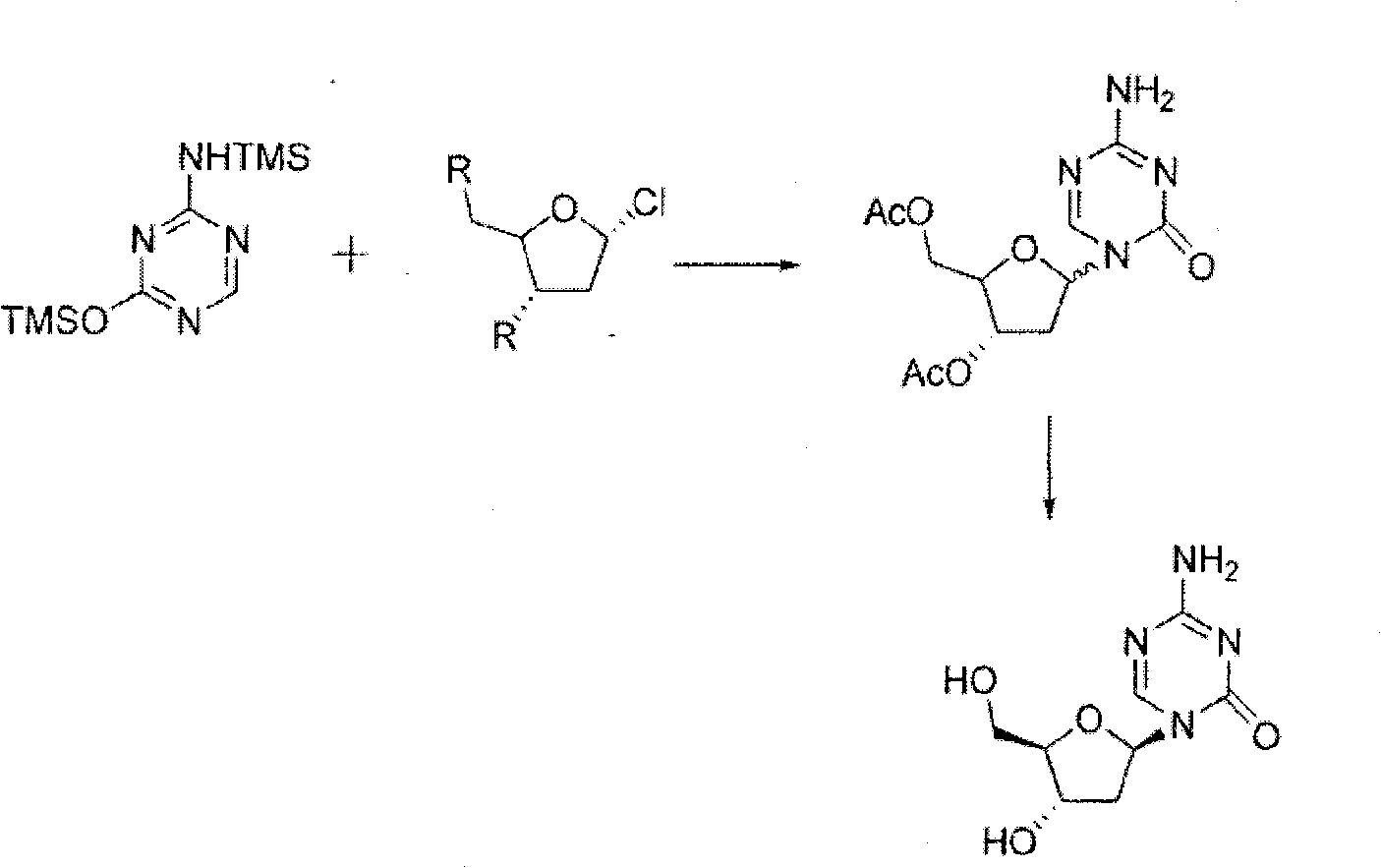
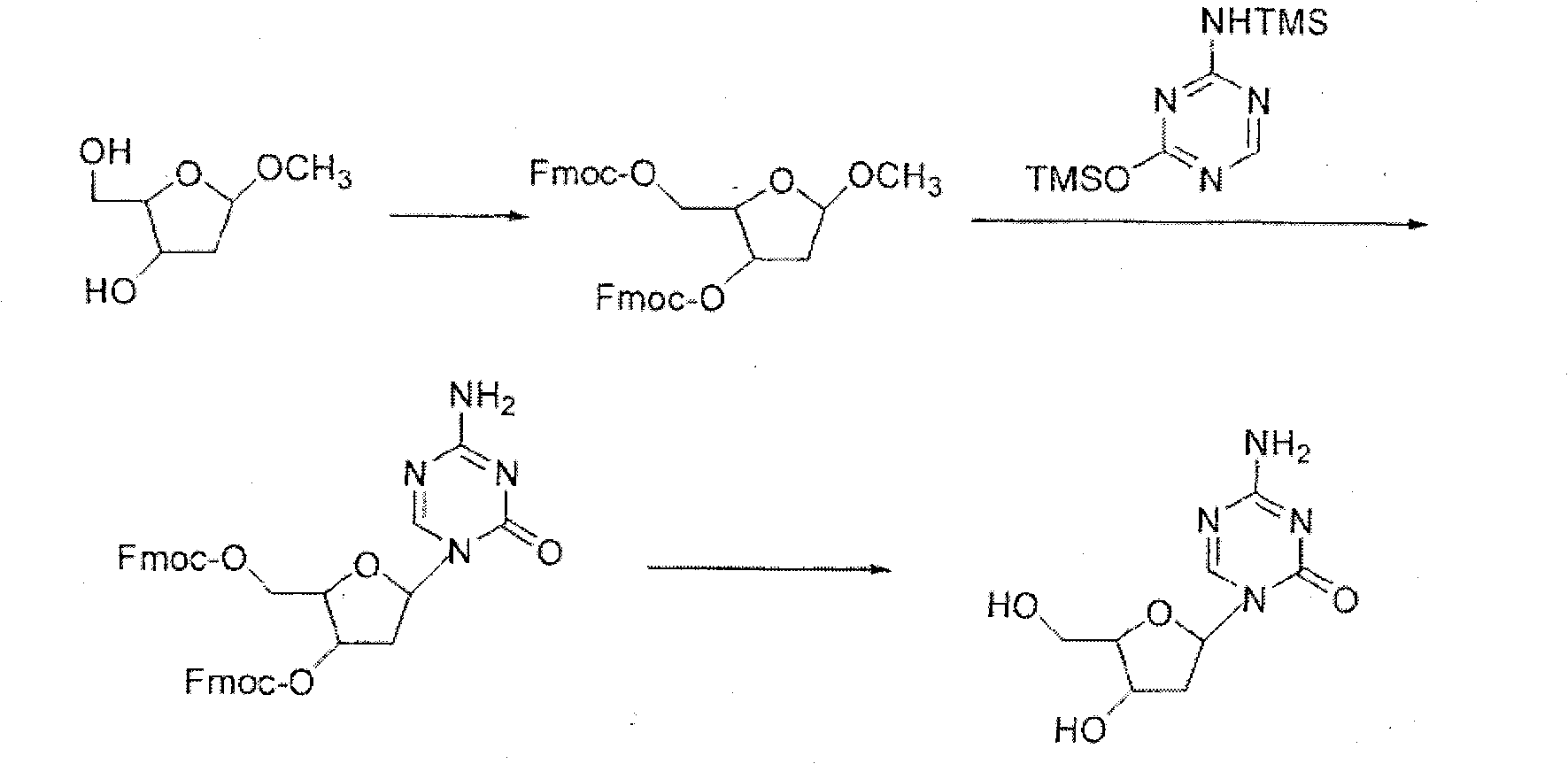
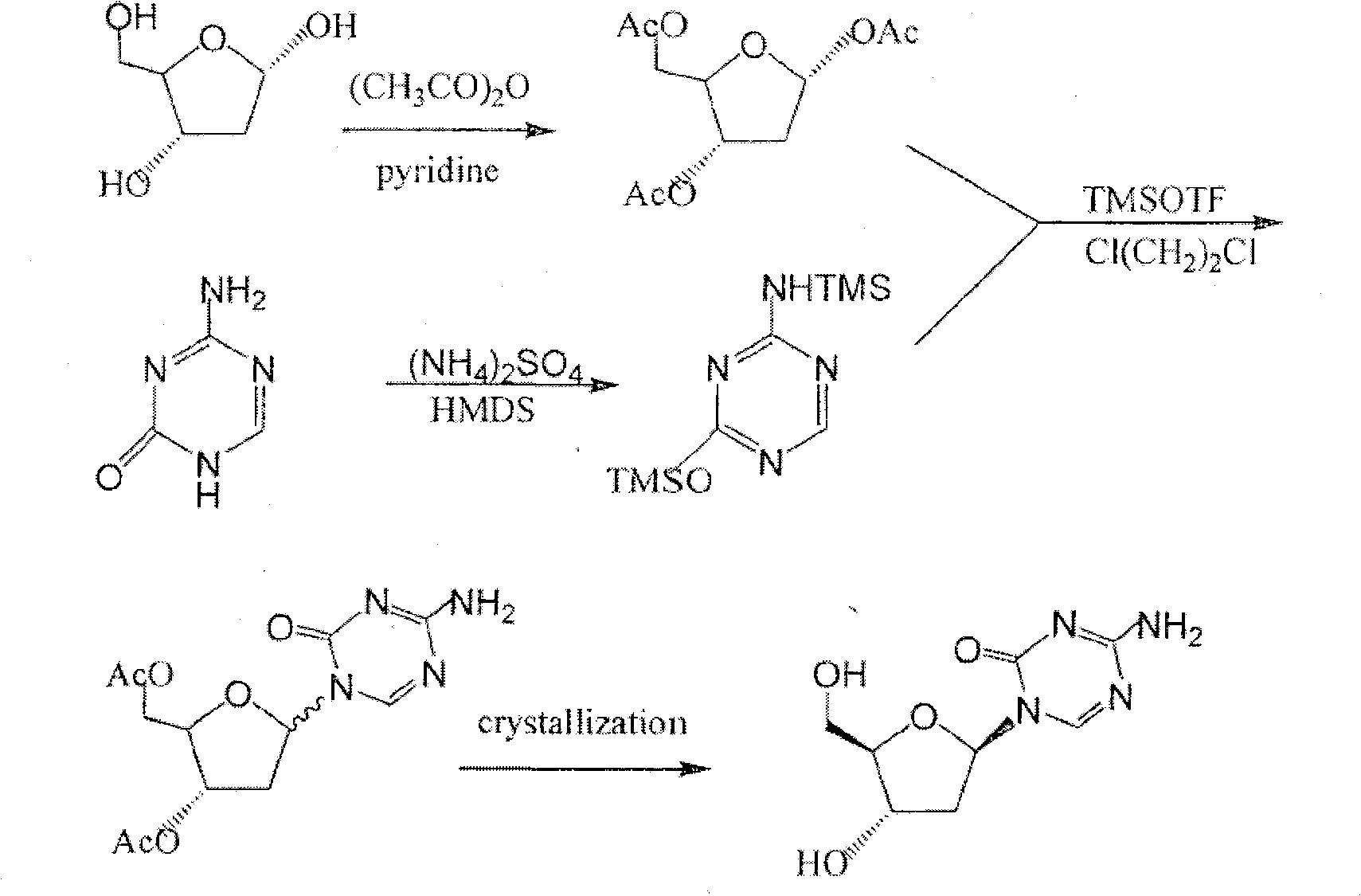
Decitabine synthesis and industrial production method
A technology of decitabine and ribose, which is applied in the field of compound preparation, can solve the problems of excessive heavy metals, low yield, excessive burning residues, etc., and achieve the effect of avoiding excessive heavy metals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step 1 (cycling)
[0043] In a 10L reaction flask, add methanol solution (7.6L) containing 0.1% (w / v) hydrogen chloride, then add 2-deoxy-D-ribose (0.380kg, 2.83mol), stir vigorously at room temperature for 30min, and detect by TLC Control the completion of the reaction, add anhydrous pyridine, and control the pH value to 7.5-8.0. Concentrate under reduced pressure below 70°C to obtain yellow viscous intermediate 2, which is directly used in the next reaction.
[0044] Step 2 (Protection)
[0045] Put intermediate 2 (obtained in the previous step) and pyridine (6.0L) into a 10L reaction flask, stir until dissolved, cool to 0°C, carefully add fluorenylmethoxycarbonyl chloride (1.520kg, 5.87mol), and the addition is complete. Stop cooling, naturally warm up to room temperature, keep the room temperature and continue to react for about 3 hours, TLC detects that the reaction is complete, extract with diethyl ether (5L) and water (5L), separate the organic phase, and extra...
Embodiment 2~12
[0058] Preparation of 2-deoxy-1-α,β-(4-amino-1,3,5-triazin-2-1(H)-on-1-yl)-3,4-di-(O-Fmoc) -D-ribose (intermediate 5)
[0059] Operation is the same as in Example 1, and the results are shown in Table 1, Table 2 and Table 3.
[0060] The impact of table 1 intermediate 4' and intermediate 4 molar ratio on yield and isomer ratio
[0061]
[0062] The impact of table two TMSOTf and intermediate 4 molar ratio on yield and isomer ratio
[0063]
[0064]
[0065] The impact of table three reaction time on yield and isomer ratio
[0066]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com