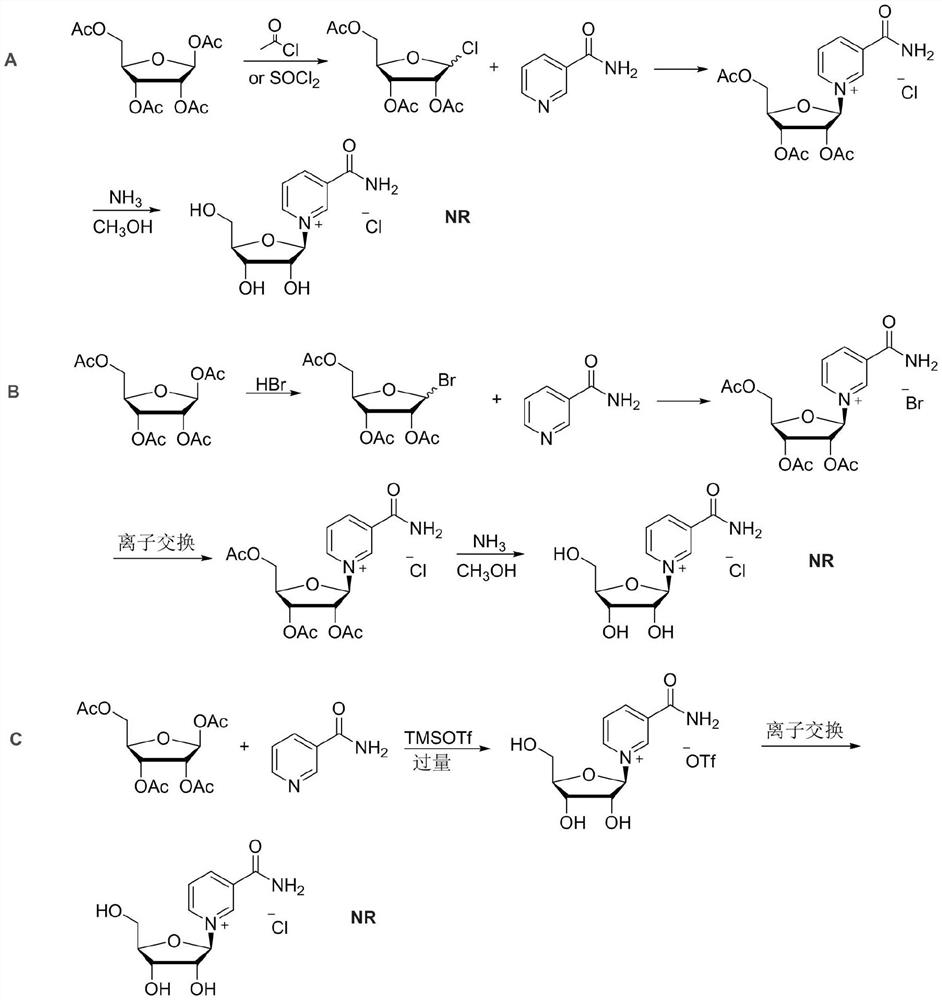
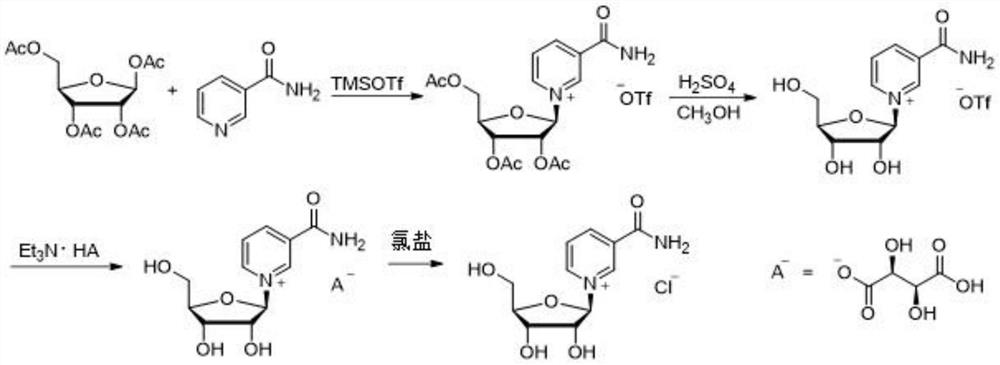
Synthesis method of nicotinamide chloride ribose
A technology for the synthesis of nicotinamide chloride and its synthesis method, which is applied in the field of synthesis of nicotinamide ribose chloride, which can solve the problems of hydrogen chloride corrosion on equipment, easy decomposition, and inability to remove trifluoromethanesulfonate groups, and achieve the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A synthetic method for nicotinamide riboside chloride, comprising the steps of:
[0026] (1) Take 600ml of acetonitrile and add it to a 1000ml round bottom flask with magnetic stirring. Under the protection of nitrogen, add 12.21g (0.10mol) of nicotinamide and 31.83g (0.10mol) of β-D-tetraacetylribose. 18.76ml (0.10mol) of trimethylsilyl trifluoromethanesulfonate was added dropwise, and stirring was continued for 40 minutes after the dropwise addition was completed, then acetonitrile was removed by distillation under reduced pressure at 35°C, and 200ml of dichloromethane and 3 gram of activated carbon, filtered after stirring for 20 minutes, and obtained nicotinamide-2,3,5-tri-O-acetyl-β-D-ribose trifluoromethanesulfonate after vacuum distillation of the resulting filtrate at room temperature to remove dichloromethane Solid 53.04 g (0.10 mol), yield 100%.
[0027] (2) Dissolve 53.04 g (0.10 mol) of nicotinamide-2,3,5-tri-O-acetyl-β-D-ribose trifluoromethanesulfonate in...
Embodiment 2
[0030] A synthetic method for nicotinamide riboside chloride, comprising the steps of:
[0031] Steps (1) and (2) are the same as in Example 1, and step (3) is: dissolve 24.26 g (0.06 mol) of nicotinamide-β-D-ribose L-bitartrate in 80 ml of deionized water, add hydroxide Sodium 2.40g (0.06mol), stirred at room temperature for 30 minutes and then cooled to 0°C, added 7.99g (0.072mol) of calcium chloride, continued to stir for 2 hours at 0°C, filtered out the precipitated L-calcium tartrate after the reaction Precipitation, dried and stored separately; the filtrate was lyophilized, and the solid obtained after lyophilization was dissolved in 60ml of ethanol, stirred evenly, and the precipitate was filtered off, and the obtained filtrate was distilled under reduced pressure to remove ethanol to obtain 15.35 grams (0.088mol) of nicotinamide ribose chloride as a solid. The purity is 99.4%, and the yield is 87.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com